Presentation: The patient with something subtle that you DON'T want to overlook
Title: Pituitary
Date & Time: Friday, January 22, 2010 at 10 AM
Lecturer: Ed Friedlander MD
|
|
|
|
{14945} anterior (A) and posterior (B) pituitary
{02294} anterior pituitary, histology; curious stain, don't worry about the colors
{14948} posterior pituitary, histology (arrow: Herring body)
QUIZBANK
Endocrine (It's impossible to separate pituitary, adrenal, thyroid, parathyroid, etc. Look at it all now.)
INTRODUCTION
Pituitary disease, like other endocrine disease, is fairly common, and is generally treatable if (and only if!) you discover it. Overview of the surgical pathology of the pituitary gland ("practical pituitary pathology"): Arch. Path. Lab. Med. 132: 1231, 2008.
The ADENOHYPOPHYSIS ("anterior pituitary") produces ACTH, TSH, FSH, LH, GH, and prolactin. No need to review these (or even what they stand for), beyond mentioning that if the input from the hypothalamus is interrupted, the adenohypophysis makes more prolactin and less of everything else.
HYPERPITUITARISM is defined as too much of one (or maybe two) of the hormones from the adenohypophysis. This may be due either to autonomous over-production (i.e., from a primary adenoma here, true adenocarcinoma of the adenohypophysis very rare: Cancer 79: 804, 1997, J. Neurosurg. 96: 352, 2002), from excess production of hypophyseal stimulating factors or underproduction of inhibiting factors, or loss of inhibition following destruction of other endocrine glands.
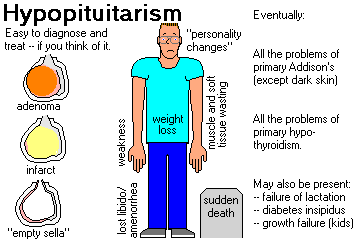
HYPOPITUITARISM ("Simmonds's disease") is defined to mean loss of one or more (often all) of the hormones from the adenohypophysis. You need to lose about 75% of the mass of the adenohypophysis before any clinical changes occur. PANHYPOPITUITARISM indicates loss of most or all of the hormones of the adenohypophysis.
The illness is underdiagnosed and deadly (Lancet 369: 1461, 2007).
In generalized anterior pituitaryfailure, loss of the hormones follows a predictable sequence. First growth hormone goes ("I don't feel good"), then FSH/LH ("My libido is gone"), then TSH and ACTH (danger to life).
The NEUROHYPOPHYSIS ("posterior pituitary") is composed of the axons of hypothalamic neurons. The "pituicytes" are modified glial elements. You may notice "Herring bodies" here, composed of ADH and maybe oxytocin awaiting release. (The other hormone secreted here is, of course, oxytocin).
The PARS INTERMEDIA, which seems to be vestigial in humans, consists of a few colloidal cysts that look like thyroid gland, but are actually remnants of Rathke's pouch and the presumed origin of the craniopharyngioma. The pars intermedia contains some odd breakdown products of the endorphin-and-ACTH precursor molecule. It's long been known that the pars intermedia enables frogs and chameleons change skin color; now we know this is done by chromogranin (Endocrinology 140: 4104, 1999).
You also know that the pituitary itself lies in the "sella turcica" (Turkish saddle) of the skull, with an extension of dura (the "diaphragma sellae") serving as the roof. The pituitary stalk passes through a hole in the middle of the sella. The pituitary gets its blood via a portal system from the hypothalamus, which carries the various brain hormones that control its function.
LABS TO LOOK FOR PITUITARY INSUFFICIENCY
To confirm endocrine insufficiency, you'll usually perform a STIMULATION TEST. In other words, you'll do something that should make the patient produce the hormone in question. If enough of the hormone does not appear in the blood, you have confirmed that disease is present.
I suggest you leave the arcane tests to the endocrinology consultant. Here's a what's-worth-knowing account for each pituitary hormone.
NOT ENOUGH ACTH... Careful!
"CRH test": Give corticotropin releasing hormone by vein. Look for an appropriate increase in ACTH and cortisol.
"Insulin tolerance test": Give insulin to drop the blood glucose below 40 mg%. Look for an appropriate increase in serum cortisol.
* "Metyrapone test": Give metyrapone, which blocks conversion of 11-deoxycortisol into cortisol, at midnight. This renders the patient unable to make cortisol and if the pituitary kicks in as it should and there's a good adrenal, there should be a great deal of 11-deoxycortisol in the blood in the morning. Also measure plasma cortisol to see if the patient took the medicine and has good adrenals. I cannot recommend this test to screen for pituitary insufficiency.
"ACTH stimulation test": Give ACTH and see if you get a cortisol and aldosterone response. Of course this is really testing the adrenal gland's ability to respond, but if the pituitary hasn't been making ACTH, the adrenal will be atrophic. You can repeat the test over the next few days, and if the response is better each time, the problem is in the pituitary (why?); if not, the problem is in the adrenal (why?)
Catheter artists sampling petrosal sinus blood for ACTH levels ("Where's that tumor?") may give CRH beforehand (update on the technique: J. Clin. Endo. Metab. 91: 221, 2006).
NOT ENOUGH ANTI-DIURETIC HORMONE
Restrict water for a while, perhaps overnight and/or until the patient is uncomfortable. If the urine does not rise above 1.010 SG / 300 mOSM/L, you have diabetes insipidus. To see whether ADH deficiency is the cause (rather than a kidney tubule problem), give an injection of ADH (as desmopressin).
Hardcore physicians may administer hypertonic saline to get the test over with, and/or assay plasma ADH before and during the procedure.
NOT ENOUGH FSH, LH
"GnRH test": If you must do a stimulation test, this is the way to see whether FSH and LH can be raised.
"Arginine test": A large amount of arginine is infused slowly by vein.
"GHRH test": Growth hormone releasing hormone is given by vein.
"Insulin tolerance test": Give insulin to drop the blood glucose below 40 mg%.
"L-DOPA test": A big dose of L-Dopa is given orally.
"TRH test": A dose is given by vein. This should greatly increase TSH levels. Same warning as before.
TOO MUCH ACTH
"High dose dexamethasone suppression test": 8 gm of dexamethasone is usually enough to suppress an ACTH-producing pituitary adenoma and the cortisol production that results from it. Other protocols exist. Of course, this has no effect on an oat cell carcinoma, thymoma, or carcinoid that is pumping out ACTH and/or CRF, or on an adrenal cortical adenoma that is putting out cortisol autonomously.
* Midnight salivary cortisol to screen for cushingism: J. Clin. Endo. Metab. 89: 3345, 2004.
TOO MUCH ANTI-DIURETIC HORMONE
Please don't order a plasma ADH if you're looking for SIADH.
ANTERIOR LOBE ADENOMAS (NEJM 324: 822, 1991; curious five-tier WHO classification Cancer 78: 502, 1996; tumorigenesis J. Clin. Inv. 112: 1603, 2003)
{15683} pituitary adenoma, gross
{15682} pituitary adenoma with hemorrhage
{49422} pituitary adenoma, gross
{49612} pituitary adenoma, gross
{09214} pituitary adenoma, histology (this was a prolactinoma; you couldn't tell)
{24821} pituitary acidophilic adenoma, Orange G stain (acromegaly)
{09215} pituitary adenoma, histology
{15679} pituitary adenoma, histology
{05026} pituitary adenoma, x-ray
{00344} pituitary adenoma, x-ray
Pituitary adenomas constitute 10% of all diagnosed primary intracranial tumors. They can occur at any age, with no great sex predominance. MICROADENOMAS are less than 1 cm; MACROADENOMAS are 1 cm or more.
They are more common in patients with autosomal dominant multiple endocrine neoplasia (MEN) I syndrome (gene MENIN) and the less-common Carney complex.
If there are other pituitary-adenoma family syndromes, they are rare (J. Clin. Endo. Metab. 91: 3316, 2006).
While the etiology of these tumors is almost entirely unknown, current work confirms clonality, at least for prolactinomas (Cancer 95: 258, 2002), i.e., regardless of what hormonal factors at work, they are true tumors.
The classic teaching is that "almost all pituitary tumors of glandular origin are benign." Today, however, we distinguish the majority of pituitary adenomas (which are treated successfully by the classic trans-sphenoidal resection) from the 35% or so that are "locally invasive" and probably can't be resected fully without causing brain damage (Postgrad. Med. 121: 168, 2009) and must be radiated and/or treated medically.
Most of these tumors are not completely autonomous, and remain to a limited extent under feedback control of the usual non-pituitary hormones.
* An oncogene PTTG is known (J. Clin. Endo. Metab. 84: 761, 1999). However, unlike other common tumors, there's little uniformity in the known genetics of pituitary adenomas from patient to patient: J. Clin. Path. 56: 561, 2003.
Around 40% of pituitary tumors from acromegalics have activation, by mutation, of gene for the alpha-chain of Gs, (oncogene gsp), the stimulatory regulator of adenyl cyclase (J. Clin. End. Met. 71: 1416, 1990; J. Clin. End. Metab. 83: 1604, 1998; Cancer 72: 1386, 1993; J. Clin. Invest. 91: 2815, 1993).
Pituitary adenomas typically present as one or more of the following:
(1) endocrine problems, both from hormones produced by the tumor itself and from damage to the rest of the adenohypophysis and/or the neurohypophysis;
(2) visual problems, from an expanding mass impinging on the optic chiasm (i.e., bitemporal hemianopsia; "Big Robbins" contains an error here) or optic nerves (blindness in one eye);
(3) enlarged sella turcica on skull x-rays, again due to expanding masses;
(4) least often, signs of increased intracranial pressure (i.e., headache, nausea and vomiting).
Large pituitary adenomas eventually erode the sella, clinoid processes, diaphragma sellae, optic nerves and chiasm, and even the cavernous sinuses, nasal sinuses, or brain.
Despite this local destruction, cancers (i.e., metastasizing tumors) of the adenohypophysis are very rare, and the presence of metastases is the only criterion for malignancy.
Infarction / hemorrhage into a large pituitary adenoma can produce PITUITARY APOPLEXY, which can simulate a berry aneurysm rupture. Large tumors may also infarct themselves, leading to remission or destruction of the remaining normal gland as well, a less dramatic clinical picture. Updates J. Neurosurg. 104: 931, 2006; J. Neurosurg. 108: 909, 2008.
Microscopically, pituitary adenomas are typical endocrine adenomas, i.e. they are composed of cuboidal cells, with round nuclei and a good blood supply.
ACIDOPHILIC ADENOMAS (eosinophilic adenomas) typically make growth hormone and/or prolactin.
BASOPHILIC ADENOMAS typically make ACTH; less often, they make TSH or the gonadotropins.
CHROMOPHOBE ADENOMAS typically make prolactin or "nothing" ("null cell adenoma", * stains as a apudoma).
However, there are many exceptions. We recommend immunostaining over reliance on either H&E, other histochemical stains, or electron microscopy.
We would like you to know the approximate frequencies of production of various hormones by pituitary tumors:
Prolactin... 30%...Men: impotence, loss of libido; Women: amenorrhea, galactorrhea, infertility; Both: Obesity (Acta Endo. 125: 392, 1991)
Growth hormone...30%... Children: gigantism; Adults: acromegaly (* may be silent: Am. J. Path. 134: 345, 1989). Many of these also make some prolactin.
ACTH... 15%... Cushing's disease
LH, FSH... 25%?... Many of these make only the beta-chains, and are not hormonally active
Most are silent until mass effect or autopsy makes the tumor known. NOTE: FSH and LH production by pituitary adenomas was, until recently, considered "rare". Now it is clear, thanks to improved staining (Am. J. Clin. Path. 106: 16, 1996) and clinicians' studies, that many (if not most) "non-functioning" pituitary adenomas will produce FSH and/or LH (or some subunit thereof), at least if TRH is given for stimulation (NEJM 324: 589, 1991). Review Mayo Clin. Proc. 71: 649, 1996.
TSH... uncommon but by no means unknown Hyperthyroidism (Ann. Int. Med. 111: 827, 1989; South. Med. J. 96: 933, 2003; J. Clin. Endo. Metab. 93: 4052, 2009)
None... ??
* Pancreastatin... An obscure hormone still in search of a disease, found in most or all non-prolactin-producing pituitary adenomas; derived from chromogranin, it inhibits insulin and PTH production (Am. J. Path. 148: 2057, 1996).
PROLACTINOMAS ("lactotrophic adenomas") are typical benign endocrine tumors, often with large eosinophilic cells.
Real functioning prolactinomas in women generally declare themselves while quite small. But remember that there are many other causes of hyperprolactinemia, including hypothalamic tumors (* I.e., craniopharyngiomas, gliomas, hypothalamic germinomas) and anti-dopamine drugs (phenothiazines, reserpine, alpha-methyldopa -- common). Try to demonstrate a mass before operating.
We've recently noticed that about 9% of people with big prolactinomas have CSF rhinorrhea (J. Clin. Endo. Metab. 92: 3829, 2008) not the result of surgery. Again, please don't miss this.
The treatment of choice for prolactinomas is the dopamine agonist bromocriptine with or without surgery (Am. J. Dis. Child. 144: 20, 1990) or gamma knife; bromocriptine almost invariably causes some significant tumor regression. Newer agents are available including cabergoline (now widely used: J. Clin. Endo. Metab. 86: 5256, 2001; J. Clin. Endo. Metab. 89: 1704, 2004).
After a few years on treatment, these tumors may regress and the bromocriptine is no longer necessary: J. Clin. Endo. Metab. 87: 3578, 2002. Future pathologists: expect a high N/C ratio and/or fibrosis in a medically treated prolactinoma (why)?
* Note that the treatment of choice for all other kinds of anterior pituitary adenomas is probably still surgery. Gamma knife radiosurgery / stereotactic radiation therapy is also coming into use, and there is now considerable experience (Cancer 110: 854, 2007).
GIGANTISM AND ACROMEGALY (NEJM 322: 966, 1990):
{49419} giant and her sisters
{16101} acromegaly
{25668} acromegaly (which twin has it?)
{49421} acromegaly, hand (compared with normal)
|
Excess growth hormone before puberty produces excessively tall stature. In the past, these people typically were crippled by nerve, muscle, and joint problems, acquired acromegalic features as they got older, and died young of complications of their diabetes. A giant is defined to be a human over seven feet tall (you may hear 200 cm instead). |  George Muresan |
Excess growth hormone after puberty produces acromegaly.
The typical acromegalic has a huge jaw ("prognathism"), huge brows, huge tongue, and huge hands (with "spade fingers"), develops a deep guttural voice, gets an oily skin (extra sebaceous glands), gets joint deformities (if not frank arthritis), and suffers from secondary diabetes and often sleep apnea.
The popular wisdom is that acromegalics are around 2.5 x more likely to develop tumors (benign, malignant) than their counterparts (Arch. Int. Med. 151: 1629, 1991). Lately this hasn't held up (J. Clin. Endo. Metab. 85: 3417, 2000).
Acromegaly kills its victims, taking an average of 10 years off life. Effective treatment of the adenomas brings overall mortality back to levels similar to the unaffected (J. Clin. End. Metab. 83: 3409, 1998).
* Growth hormone is of course popular among bodybuilders and "anti-aging" cultists, who obtain it though it is not medically indicated (JAMA 299: 2792, 2008 reviews the genuine indications -- adult growth hormone deficiency needs a proper workup; JAMA now suggests stimualtion testing and a hGH level).
The lab workup of acromegaly is straightforward. Your screening test is a spot blood insulin-like growth factor I (IGF-I). If this is normal, you have ruled out acromegaly. Next, attempt suppression of the hGH levels to <1 microgram/L by administering 75 mg of glucose orally (yuck). If this fails, your patient probably has acromegaly. |  Jaws |
Most pituitary giants and acromegalic patients have a pituitary macro-adenoma (i.e., >10 mm). The best treatment is still surgical removal of this adenoma, though some inoperable patients get fair results with the somatostatin analog octreotide (J. Clin. End. Metab. 87: 4554, 2002; update J. Clin. Endo. Metab. 90: 1856, 2005, also J. Clin. Endo. Metab. 91: 1397, 2006).
The classic "acidophilic adenomas" are "densely granulated", which gives a better surgical prognosis. Many of these patients have "sparsely granulated / chromophobe" adenomas instead. There's no correlation between growth hormone levels and tumor type; which type responds best to which medical therapies is presently under study.
The big news in pituitary disease during the last few years has been the application of the long-acting somatostatin analogue octreotide ("Sandostatin") to the treatment of acromegaly. This often shrinks the tumor, and seems to make these tumors physically softer, making it easier to remove them surgically (J. Clin. End. Metab. 86: 2779 & 5194, 2001, J. Clin. Endo. Metab. 90: 440 & 1588, 2005; lots more). A long-acting depot form is available.
Radiation is reserved for those who have failure with surgery (J. Clin. Endo. Metab. 88: 3105, 2003).
Not surprisingly, the new transgenic mice that overproduce human growth-hormone releasing factor get hyperplasia and adenomas of growth hormone and prolactin producing cells (Endocrinology 131: 2083, 1992), and morphologic changes elsewhere resembling those in acromegaly (Am. J. Path. 141: 895, 1992).
* There are a couple of poorly-understood "acromegaloid" syndromes, with the acromegaly look and the insulin resistance, but normal growth hormone levels. Some of these folks have elevated levels of other growth factors. There is also a familial tumor syndrome, 11q13, the gene not yet cloned, which produces only GH-omas (J. Clin. Endo. Metab. 86: 542, 2001; gene still not isolated J. Clin. Endo. Metab. 90: 6580, 2005).
CUSHING'S DISEASE:
{09367} Cushingism
{09368} Cushingism
{09369} Cushingism
{09370} Cushingism
{16110} Cushingism
{16111} Cushingism
{49427} Cushingism
{25669} Cushingism, before and after treatment
You are already acquainted with the signs of Cushingism. "Cushing's disease" implies production of ACTH by a pituitary tumor, usually a small microadenomas. There is usually not enough ACTH production to darken the skin.
While the role of CRF in producing these adenomas may be important, they do appear to be monoclonal overgrowths (J. Clin. End. Met. 75: 472, 1992) that have lost some, but not all, of the ability of high levels of cortisol to inhibit their growth (Endo. Rev. 20: 136, 1999).
* One famous fact about these microadenomas is that they're so small and mushy that they are likely to wash out before the surgeon is even able to find them. The ACTH levels drop after surgery, though, letting everyone know what's happened.
* Transgenic mice (two kinds) with Cushing's disease: Am. J. Path. 140: 1071, 1992.
As you know, most cases of "idiopathic adrenal hyperplasia" were really due to ACTH-omas. In the old days when we'd remove both hyperplastic adrenals, the ACTH-oma, which was under some feedback inhibition, would grow to a tremendous size, turn the patient's skin brown, and blind and kill the patient ("Nelson's syndrome"). Establish your diagnosis and take the pituitary out instead.
Back in the Bad Old Days, if you got an ACTH-producing pituitary adenoma, you would probably be really sick for a while and then die of it. Today you will probably get a cure (Ann. Int. Med. 130: 821, 1999); however persistence / recurrence is disturbingly common (J. Clin. Endo. Metab. 89: 6348, 2004).
* Future catheter artists: Even today, it's often hard to find the adenoma on imaging studies, especially in younger folks (J. Clin. Endo. Metab. 90: 5134, 2005). It's long been a practice to find and lateralize non-visualized adenomas by comparing simultaneous ACTH measurements in the petrosal sinuses (see, for example, Am. J. Ob. Gyn. 171: 563, 1994). This is being applied to more diseases (Am. J. Med. 87: 679, 1989; NEJM 324: 822, 1991).
SECONDARY HYPERTHYROIDISM
Mysteriously (and fortunately), TSH-producing adenomas of the pituitary gland are quite rare. They tend to look a little bit anaplastic and to be locally aggressive.
"Pituitary hyperplasia due to resistance to thyroid hormone" (J. Clin. End. Met. 75: 1071, 1992) isn't well-understood. Patients have excess hTSH and become hyperthyroid.
* Future pathologists:
INCIDENTALOMAS: It's been known for decades that in maybe 30% autopsies on grown-ups, if you look really hard, you'll find a "pituitary microadenoma", generally "prolactin-producing". (One recent series of old autopsy pituitaries, without a diligent search of each, found 10% with 1 "adenoma", 1% with 2 or more: J. Neurosurg. 74: 243, 1991; we're now using our high-resolution MRI's to find that around 10% of live folks have them, too: Ann. Int. Med. 120: 817, 1994; Cancer 101: 613, 2004). Just how "autonomous" these "tumors" are, and their real status as "neoplasms", is very dubious; the one's I've seen look like typical endocrine-gland hyperplastic nodules ("gland bumps"). Nowadays they're routinely called "incidentalomas"; if there are no hormonal abnormalities, leave them alone, even if the pituitary seems a bit large (J. Clin. Endo. Metab. 86: 3009, 2001).
Clinicians are now picking these up on NMR scans and learning to ignore them, except for the rare ones over 10 mm (Ann. Int. Med. 122: 925, 1990; Arch. Int. Med. 155: 181, 1995).
Sometimes telling microadenoma from normal gland or hyperplastic is tricky. Microadenomas will lack the normal reticulin pattern of pituitary gland, and have homogeneous cells (morphology, immunostaining). Hyperplastic glands retain normal reticulin but the cell groups are larger.
Occasionally, secondary prolactin production is caused by the tumor blocking the portal system, keeping dopamine from reaching the non-neoplastic gland.
Most of the pituitary hormones can be secreted by carcinoids and oat-cell carcinomas elsewhere in the body.
Some pituitary adenomas are eosinophilic not because they derive from GH- or prolactin-producing cells, but because they are packed with mitochondria. You've seen these "oncocytomas" or "Hürthle cell adenomas" elsewhere.
Nowadays, some pathologists diagnose ATYPICAL PITUITARY ADENOMA when the Ki-67/MIB-1 labeling exceeds 3% and the nuclei stain with p53. They're much more likely to recur, but really aren't cancer.
Pituitary carcinomas are very rare, and no one knows exactly how to distinguish them from adenomas. So far, what has been most helpful is immunostaining for Ki-67 and p53 (J. Clin. Endo. Metab. 90: 3089, 2005).
PANHYPOPITUITARISM (NEJM 330: 1651, 1994)
The symptoms of panhypopituitarism are highly variable.
Growth hormone doesn't have spectacular effects on adults, and for years, we taught that its impact
is negligible. Now we know this isn't true. It slows the body wasting that occurs in old age (NEJM
323: 1, 1990). Adults lacking growth hormone have more fat and less muscle per pound of body
weight (Am. J. Clin. Nutr. 55: 918, 1992); the heart may also actually be weaker (Br. Heart. J. 67:
92, 1992), and growth hormone also helps maintain the skeletal muscles. See below.
Loss of gonadotropins produce loss of libido and body hair.
* There's an idiopathic, adult-onset loss of gonadotropin that produces treatable male infertility
NEJM 336: 410, 1997.
Loss of prolactin prevents lactation, which would only be noticed after childbirth.
Loss of thyrotropin produces secondary hypothyroidism (i.e., cretinism in children, problems
culminating in myxedema in adults).
Loss of ACTH produces secondary adrenal insufficiency, which is just as deadly as primary adrenal
insufficiency. (Fortunately, this happens only very late in the progression
of the disease.)
If the posterior pituitary gland is involved, loss of ADH produces pituitary diabetes insipidus.
Patients with hypopituitarism from a variety of causes are unusually prone to precocious
atherosclerosis. The
premature cardiovascular disease mortality has been known for
decades (Lancet 336: 285, 1990). One robust finding is that
"adult-onset growth hormone deficiency" (see below) is a risk factor for atherosclerosis,
and now it appears that replacing it helps prevent / reverse the process
(J. Clin. Endo. Metab. 93: 3416, 2008).
Most of the time, hypopituitarism is due to (1) destruction of the pituitary by a pituitary adenoma, surgery, radiation (Q. J. Med. 70: 145,
1989), or trauma;
(2) Sheehan's pituitary necrosis, or
(3) the "empty sella syndrome".
Also worth considering are vascular problems (i.e., cavernous sinus thrombosis, obvious) and
sarcoidosis (not at all obvious).
LYMPHOCYTIC HYPOPHYSITIS is a rare cause of pituitary insufficiency and a mass lesion; the
traditional teaching is that most patients
are postpartum women but more recently sex ratios have been about equal
(J. Neurosurg. 101: 262, 2004). As a cause of sudden death: AMFJP 30: 61, 2009.
GRANULOMATOUS HYPOPHYSITIS produces a mass and sometimes
pituitary insufficiency: J. Neurosurg. 71: 681, 1999; I've seen a case. Both
are likely to be be operated as
"adenomas" and the correct diagnosis established later by the pathologist; one must be
extremely alert to suspect the diagnosis before the (? unnecessary) surgery
(J. Clin. Endo. Metab. 86: 1048, 2001).
For some reason, the disseminated histiocytic diseases (Langerhans cell histiocytosis,
Erdheim-Chester, Rosai-Dorfman) are prone to attack the adenohypophysis as well.
Some grown men with "idiopathic hypogonadotropic hypogonadism"
have autoantibodies against the gonadotropin secreting cells of the
pituitary (J. Clin. Endo. Metab. 92: 604, 2007).
* A genetic syndrome lacks a transcription factor needed for making growth-hormone, prolactin, and
TSH
(Science 257: 1118, 1992.)
* Yet another mutation, lack of PROP1, prevents production of all the hormones
of the adenohypophysis except for (sometimes) ACTH (Nat. Genet. 18: 147, 1998;
J. Clin. Endo. Metab. 85: 4566, 2000; J. Clin. Endo. Metab. 89: 5256, 2004).
POU1F prevents productions of GH, prolactin, and TSH (J. Clin. Endo. Metab. 90:
4762, 2005). Even ACTH usually is insufficient: J. Clin. Endo. Metab. 89: 5256, 2004.
Hypopituitarism after TB SHEEHAN'S PITUITARY NECROSIS ("postpartum pituitary necrosis") occurs when shock complicates a
problem delivery. The drop in blood pressure results in inadequate blood supply to the gland, which
is already hyperplastic and has its vessels squeezed half-shut. Hence, it undergoes watershed infarction.
Less often, pituitary necrosis results from sickle cell disease, temporal arteritis, or trauma.
* For some reason, diabetics are prone to hemorrhages and necrosis in the pituitary. This may even
help the diabetic state.
Usually the gonadotropins and prolactin are the most decreased. The typical Sheehan's patient fails
to lactate or resume menstruating after delivery. When necrosis is less complete, the disease tends to
progress "as the remaining cells are entrapped in scar tissue" (more likely, the scar diverts blood
from the good gland).
The EMPTY SELLA SYNDROME classically results from slow crushing of the gland by CSF pressure when
the hole in the diaphragma sellae is wide enough (or there is another defect) to allow arachnoid to
herniate into the sella (most common).
Recent work confirms the traditional wisdom that "empty sella" usually
results from increased intracerebral pressure (J. Neurosurg. 103: 831, 2005).
Oh... the authors also remind referring physicians how embarrassing it is to miss
CSF rhinorrhea as the cause.
Some of these patients develop pituitary insufficiency.
Future radiologists: Other reasons to have an empty sella include old Sheehan's, or total necrosis of
an old adenoma, or previous surgery. Again, many of these patients have hypopituitarism of one
kind or another.
The older medical literature is full of descriptions of extreme weight loss in
end-stage hypopituitarism ("Simmonds's cachexia"). These must
be the patients who finally lose
ACTH (often the last to go). Failure to produce normal amounts of growth hormone in childhood results in miniature, well-proportioned people.
Causes
range from "idiopathic" to various genetic syndromes to other causes
of hypopituitarism.
Around 50% of "idiopathic dwarves" are breech or transverse deliveries, and the damage to the
hypothalamic-pituitary axis may occur when their little skulls get crunched: Lancet 338: 480, 1991
After traumatic brain injury, there is often considerable loss of
growth hormone (Arch. Phys. Med. 86: 463, 2005). New studies of professional
and elite amateur boxers show that growth hormone and ACTH are often
impaired -- watch this one (Ann. Int. Med. 148: 827, 2008.)
Laron dwarves (short, frontal bossing;
the defect is in the
growth hormone receptors;
update J. Clin. Endo. 83: 4481, 1998).
Quite a few adults who are "just short" turn out to have marginal
ability to make growth hormone, and they seem to benefit
from replacement (J. Clin. Endo. Metab. 89: 1586, 2004).
Pygmies also have tissues that do not respond well to growth hormone;
apparently in Africa and the Philippines there is a relative deficiency
of the growth hormone receptor ("pituitary dwarfism type II";
J. Ped. Endo. 15: 269, 2002; Clin. Endo. 51: 741, 1999).
"Get Shorty!" Now it turns out that a lot of just-plain-short people have minor defects in their
growth hormone receptors: NEJM 333: 1093, 1995.
If thyrotropin is normal or thyroid hormone is replaced, the children will be of normal intelligence.
If gonadotropin production is normal or sex steroids are administered, puberty should occur
normally. And please don't miss adrenal insufficiency in these kids; they continue to die of sudden
adrenal crisis in disturbingly large numbers.... (J. Clin. End. Metab. 81: 1693, 1996).
And of course, we used to donate autopsy pituitaries to make growth hormone to help these kids
attain normal height. (* Regrettably, a significant amount of this precious substance ended up at the
gym instead, as part of quack "muscle building" schemes.) Now we have recombinant hGH.
NOTE: Before you diagnose an endocrine problem, remember that mysterious "failure to grow" can
and does result from lack of parental warmth and emotional nurturing. This is more common
than
endocrine dwarfism.
ADULT GROWTH HORMONE DEFICIENCY, unheard-of a few years ago,
is now being diagnosed both in patients who've had previous pituitary
surgery or tumors, and in people who seem to waste their lean tissues and
simply do not feel or perform well. The screening test is IGF-I, which
should be low for age-and-sex-matched controls.
The patients report a spectacular improvement in sense of well-being upon
hormone replacement (Clin. Endo. 54: 709, 2001).
Especially, suspect it when the voice starts getting
higher and raspier (J. Clin. Endo. Metab. 90: 4128, 2005).
As noted above, the increased atherosclerosis risk seems reversible with
replacement. Exactly how common this really is gets widely debated --
the real question is, "Who's likely to benefit from the expensive replacement therapy?"
CRANIOPHARYNGIOMA ("adamantinoma", "ameloblastoma", both named for tooth enamel)
{15685} craniopharyngioma, gross
This is a benign tumor of Rathke's pouch remnants that generally occurs just above the pituitary
and sella turcica. It is locally aggressive but does not metastasize (* like the closely-related
ameloblastoma of the jaw). The optic nerves and chiasm, and then the hypothalamus, are damaged.
Most patients are under twenty, but no age is immune. Little is known
of the etiology or genetics (J. Neurosurg. 98: 162, 2003).
Grossly, most of these tumors usually filled with little
cysts that contain an unsavory, cholesterol-rich fluid ("machine oil").
Microscopically, the tumor usually recalls developing tooth enamel ("adamantinomatous type"),
with areas of columnar cells,
stellate mesenchyme, usually calcification, sometimes stratified squamous stuff and/or bone.
A minority of craniopharyngiomas exhibit simple squamous
epithelium and fibrous cores ("papillary growth pattern") instead.
The mainstay of craniopharyngioma treatment is surgery (series with pretty
good results 97: 3, 2002), but intratumoral injection of bleomycin
is a possibility for poor-surgical risk cases: J. Neurosurg. 84: 124, 1996.
Future pathologists: The papillary variant mostly occurs in adults, and is less aggressive (J.
Neurosurg. 83: 206, 1995). RATHKE'S CLEFT CYSTS of the pars intermedia, a common
tiny incidental autopsy finding, may sometimes be large enough
to be seen in life; must be distinguished from craniopharyngiomas.
These cysts can compromise eyesight, too (Am. J. Ophth. 119: 86, 1995;
J. Neurosurg. 102: 189, 2005).
Very rarely, a squamous cell carcinoma arises in a craniopharyngioma (Arch. Path.
Lab. Med. 124: 1356, 2000).
DIABETES INSIPIDUS (Am. Fam. Phys. 55: 2146, 1997;
Arch. Int. Med. 157: 1293, 1997).
Because the posterior pituitary gland is really processes of hypothalamic neurons, a variety of
processes can damage it. Remember:
Causes within the sella
Causes above the sella
Also remember nephrogenic diabetes insipidus, the inability of the kidney to respond to ADH (mutant
ADH receptor: Nature 359: 235, 1992; also other medullary diseases and lithium therapy).
* Dr. Roy Meadow again: Your lecturer believes that the (overzealous and now-disgraced)
"discoverer of Munchausen's by proxy" was correct in recognizing
salt poisoning as a means of making children sick (Arch. Dis. Child. 68: 448, 1993).
However, remember that plenty of kids have diabetes insipidus
and even more have simply dehydration.
* There are two posterior pituitary gland tumors.
PITUICYTOMAS are astrocytomas arising from the pituitcytes. I've never seen one.
The GRANULAR CELL TUMOR resembles similar tumors of Schwann cell origin seen elsewhere (but see
Virch. Arch. B., 60: 413, 1991). Think of McCune-Albright.
SYNDROME OF INAPPROPRIATE ADH PRODUCTION
Patients have continual ADH production no matter what the current plasma osmolality. Water leaks back freely
from the collecting ducts, the blood becomes hypotonic, and the patient loses the ability to produce
dilute urine.
Low blood tonicity leads to seizures and then to death. Correct it too fast by pushing in sodium, and
your patient develops central pontine myelinolysis. Be sure you know what you're doing.
The syndrome, when really present, is almost always due to ectopic ADH production by a tumor
(typically, oat cell carcinoma; occasionally a carcinoid, * thymoma, or * lymphoma; rarely,
widespread pulmonary TB The best treatment is to make the "inappropriate ADH" appropriate by restricting water.
Before you wrongly diagnose "syndrome of inappropriate ADH" in your cachectic, hyponatremic
cancer patient, remember that generalized body protein depletion re-sets the "osmostat", and
hyponatremia is usual and normal. Don't add to your patient's discomfort by foolishly denying him
or her access to the water pitcher.
There is no known oxytocin-excess or deficiency syndrome. PITUITARY-HYPOTHALAMIC SYNDROMES
These result from abnormal function of the hypothalamus, reflected in problems with sexual
development.
FROEHLICH'S SYNDROME is hypothalamic hypogonadism plus obesity. Affected boys are obese (i.e.,
have increased appetite), show a female pattern of fat distribution, and have delayed (if ever)
appearance of primary or secondary sex characteristics.
The problem in "adiposogenital dystrophy" may be in the hypothalamus (true Froehlich's -- these
kids may or may not be retarded; do you think emotions could be the cause?). It can equally well be
due to hypopituitarism from any cause in someone who like to eat. It could also be "constitutional"
without a demonstrable hypothalamic lesion (the fat boy that stays child-like; you knew him).
Good
luck sorting all these out; endocrinologists use sophisticated stimulation and suppression tests.
Current thinking focuses on a variety of etiologies, known and unknown,
that prevent the normal pulsatile secretion of GnRH.
{49423} Froehlich's man, age twenty
After surgery for craniopharyngioma, which often damages the hypothalamus, MRI can predict
who, and will not, get morbid weight gain (J. Clin. End. Metab. 81: 2734, 1996).
You already know PRADER-WILLI.
* BARDET-BIEDL (used to be "Laurence-Moon-Biedl")
is a hereditary complex with retinitis pigmentosa, polydactyly,
and a similar picture to Froehlich's. There are at least three loci (Am. J. Hum. Genet. 72: 650, 2003).
An autosomal dominant Kallman's: Nat. Genet. 33: 463, 2003.
* GPR54 mutations lead to no-puberty (NEJM 349: 114, 2003).
MCCUNE-ALBRIGHT SYNDROME (often just "Albright") is a syndrome with cutaneous café-au-lait
("coffee with milk") spots (with irregular borders), polyostotic fibrous dysplasia, and precocious
puberty caused (maybe sometimes) by a curious hypothalamic hamartoma that produces LH-releasing hormone. (These
hamartomas are
infamous for causing puberty before age 2. McCune-Albright kids also have other reasons for having hormonal problems.)
Much more about McCune-Albright (a genetic disease that cannot be transmitted
parent to child) when we study bone.
* Septo-optic dysplasia, homeobox gene HESX1, multiple birth defects including
several malformations of the forebrain; there are others (Nature 403: 658, 2000).
* Update on all the genes involved in pituitary development and
neoplasia: J. Clin. Inv. 112: 1603, 2003.
* I have "HPA Axis Disease!" There's considerable research interest right now in
subtle physiological alterations of the hypophyseal-pituitary axis
as a result of life experience and/or subtle genetic differences.
This supposedly explains why some
people overeat, get post-traumatic stress disorder (J. Clin. Psych. 62S17:
41, 2001), functional GI
troubles (Am. J. Med. 107(5A): 12S, 1999), fibromyalgia,
etc., etc. Cause or effect, the results are interesting;
the axis is easy to study by stimulation and suppression tests.
Respectable psychiatrists are rediscovering the dexamethasone suppression
test (if abnormal, the patient is MUCH more likely to suicide: Am. J. Psych.
158: 748, 2001; circadian and day-to-day
rhythms distinguish various types of severe depression Arch. Gen. Psych.
57: 755, 2000).
Having been badly abused as a child seems to make a person oversecrete CRF /
downregulate CRF receptors etc., etc. (Am. J. Psych. 158: 575, 2001),
PTSD patients exhibit an enhanced ACTH suppression to dexamethasone (Am. J. Psych. 161: 1397, 2004),
and this will make "nature vs. nurture"
almost impossible to sort out for now.
"HPA axis" is now becoming a "pop" diagnosis, in a class with
chronic fatigue syndrome, fibromyalgia, and multiple chemical sensitivities
(except for the last, I think there is something real, but there are plenty of somatizers
and it is hard to sort out.)
* PITUITARY NON-DISEASES
Little pituitary INFARCTS are common in patients who die with intracranial problems.
{10747} pituitary infarct
CROOKE'S HYALINE CHANGE is seen in ACTH-producing cells, with
overly-dense cytoskeleton, sometimes with ringlike inclusions ("enigmatic bodies", giant lysosomes).
It results from
ACTH, when they have suffered chronic feedback
suppression by circulating glucocorticoids
(iatrogenic, from an autonomous adrenal adenoma, in the non-neoplastic corticortophs
in Cushing's disease, etc.)
We'll leave you to sort out the "ethical aspects" of hGH therapy.
It's fascinating, and opens into area of drug-company gouging, whether
feebleminded people should be treated, whether it's right to ask society to
pay $300,000 per pituitary dwarf, whether Mr. Stallone and the boys raised
on hGH to become taller adults (now commonplace) are right to do this,
and whether we have a duty to treat an American boy who'll probably grow up to be less than 5'6",
which will diminish his chances for marriage and getting a good job.
(No, it's not right, but it's an ugly fact of life.)
FINAL NOTE:
Endocrine disease is especially worthy of your serious attention because (1) it is prevalent; (2) it is
generally very treatable; (3) if you diagnose it incorrectly, you doom the patient to lifelong
medication; (4) if you miss it, you doom the patient to long-term ill health and probably "mental
illness"; (5) Many (if not most) cases of endocrine disease get missed for a long, long time.
You'll learn how to establish the presence of various endocrine syndromes and diseases while you
are on Internal Medicine. Again, remember that for most suspected non-thyroid endocrine
diseases, you'll need a stimulation test ("Can the patient produce the hormone in question at all?") or
a suppression test ("Can we suppress production of the hormone in question as we could in a healthy
patient?"). I don't want any more requests for a "random growth hormone assay" on a short kid.
{00135} thyroid, normal
![]() Pituitary adenoma
Pituitary adenoma
Pittsburgh Illustrated Case
![]() meningitis in childhood is a problem in the poor nations (Ann. Int. Med.
118: 701, 1993). In this series, around 1/5 of childhood survivors of TB meningitis were left with
hypopituitarism.
meningitis in childhood is a problem in the poor nations (Ann. Int. Med.
118: 701, 1993). In this series, around 1/5 of childhood survivors of TB meningitis were left with
hypopituitarism.

PITUITARY DWARFISM

{15686} craniopharyngioma, gross
{15687} craniopharyngioma, histology
{15688} craniopharyngioma, histology
![]() Craniopharyngioma
Craniopharyngioma
Notice the benign squamous pearl
KU Collection
* Future pathologists: You can tell a
adamantinomatous craniopharyngioma from an innocent
Rathke cyst because the craniopharyngioma
exhibits nuclear staining with beta-catenin.
![]()
![]() produces excess ADH for some reason, and the last one to remember is
acute intermittent porphyria and its variants). Pituitary problems almost never produce
inappropriate ADH.
produces excess ADH for some reason, and the last one to remember is
acute intermittent porphyria and its variants). Pituitary problems almost never produce
inappropriate ADH.
KALLMANN'S SYNDROME
is a brain malformation with anosmia (no sense of smell) and Froehlich's.
The best-known gene is KAL1, which directs neuronal migration; two other loci are known.
* Jazz singer/musician "Little Jimmy Scott", who's
delighted audiences for over half a century,
has Kallmann's, which gave him his distinctive child-like appearance and voice.

 * SLICE OF LIFE REVIEW: ALL GLANDS
* SLICE OF LIFE REVIEW: ALL GLANDS
{09213} pituitary, normal
{09362} thyroid scan radionucleotide, normal
{11204} adrenal and nerve, normal
{11207} adrenal and nerve, normal
{11210} adrenal and nerve, normal
{11754} thyroid, normal
{11755} thyroid, normal
{11803} thyroid, normal
{12866} sella turcica, normal anatomy
{12903} thyroid gland, normal
{12983} sella turcica, normal
{12986} sella turcica, normal
{12992} sella turcica, normal anatomy
{12995} sella turcica, normal
{12998} sella turcica, normal
{13004} sella turcica, normal
{13007} sella turcica, normal
{13010} sella turcica, normal
{13013} sella turcica, normal
{13016} sella turcica, normal
{13019} sella turcica, normal
{13022} sella turcica, normal
{13025} sella turcica, normal
{13028} sella turcica, norma
{13169} adenoma, pituitary
{14942} hypophysis, normal
{14942} hypophysis, normal
{14943} adenohypophysis (pars distalis), normal
{14943} adenohypophysis (pars distalis), normal
{14944} pituitary (anterior & posterior)
{14945} pituitary (anterior & posterior)
{14946} pituitary trabeculae, normal
{14947} neurohypophysis, normal
{14948} neurohypophysis, normal
{15034} adrenal, normal
{15035} adrenal gland (zones), normal
{15036} adrenal gland (zones), normal
{15037} adrenal gland (cortex), normal
{15038} adrenal gland (cortex), normal
{15039} adrenal gland (cortex), normal
{15040} adrenal gland (cortex), normal
{15041} adrenal gland (cortex, lipid stain)
{15042} adrenal gland (cortex, lipid stain)
{15043} adrenal gland (medulla), normal
{15044} adrenal gland (medulla), normal
{15045} adrenal gland (medulla, chromaffin stain)
{15046} adrenal gland (medulla, chromaffin stain)
{15048} thyroid gland, normal
{15049} thyroid gland, normal
{15050} thyroid inactive, normal
{15051} thyroid gland (follicle cells), normal
{15052} thyroid gland (active follicle cells)
{15053} thyroid gland (inactive follicle cells)
{15054} thyroid gland (parafollicular cells)
{15055} thyroid gland (parafollicular cells)
{15056} parathyroid gland fetal, normal
{15057} parathyroid gland, normal
{15058} parathyroid gland, oxyphil cells
{15059} parathyroid gland, oxyphil cells
{15060} parathyroid gland, oxyphil & chief cells
{15680} adenoma, pituitary with normal tissue
{20695} pituitary gland, both lobes
{20696} pituitary gland, both lobes
{20697} pituitary, pars distalis
{20698} pituitary, pars intermedia
{20699} pituitary, intermedia and * nervosa
{20700} pituitary, pars nervosa
{20701} adrenal gland with layers labeled, #98
{20702} adrenal gland with layers labeled, #98
{20703} adrenal gland with layers labeled, #98
{20704} adrenal gland with layers labeled, #98
{20705} adrenal gland, medulla
{20706} thyroid, normal
{20707} thyroid, normal
{20708} thyroid, normal
{20709} parathyroid, normal
{20711} pineal gland, normal
{20712} pineal gland, normal
{20795} parathyroid
{20796} parathyroid, oxyphil cell
{20797} parathyroid, oxyphil cell
{20970} hypophysis, all three regions
{20971} adenohypophysis, anterior lobe of pit.
{20972} neurohypophysis, posterior lobe pituit.
{20973} pars * Intermedia, pituitary
{20974} pars * Intermedia, pituitary
{20975} neurohypophysis, herring body
{20976} adrenal
{20977} adrenal, glomerulosa layer
{20978} adrenal, fasciculata layer
{20979} adrenal, reticularis layer
{20980} adrenal, medulla
{20981} adrenal, fasciculata
{24712} adrenal, normal
{24823} thyroid, normal
{25393} adrenal cortex, normal
{31090} pituitary in sella, normal
{34355} pituitary, normal
{36419} thyroid, normal
{36425} thyroid, normal
{36452} thyroid cytology, normal glandular cells
{36455} thyroid cytology, sheet of normal glandular cells