Title: Cardiomyopahties
Date & Time: Monday, February 4, 2013 at 10 AM
Lecturer: The Pathology Team
QUIZBANK
Heart (all)
INTRODUCTION
Cardiac pathology is relatively straightforward, if you understand the heart's physiology.
There are only a few important diseases and patterns of injury, and most of these are fairly well understood.
Myocardial biopsy is becoming more and more important, especially in the transplant era. All about it: Mayo Clin. Proc. 86: 1095, 2011.
Looking at pictures of the heart? Remember:
I think that the pathology of the heart presents fewer difficulties than any other organ system except GI tract, as long as you understand the physiology.
* We used to think that myocardial cells were post-mitotic. During the 1990's, it became clear that hyperplasia is a feature of the deformity in the vicious cycle of congestive heart failure. Now it's clear (from studies of nuclear fallout) that we regenerate maybe 1% of our heart fibers yearly (Science 324: 98, 2009).
A variety of genetic syndromes produce various problems with the cardiovascular system. I have tried to resist the tremendous temptation to describe all my favorites. Instead, I've included only the ones that a generalist should know.
Had CAST not included a placebo group, the erroneous conclusion that drug therapy did no harm might have been reached.
--NEJM, cited above.
"Proarrhythmias" are rhythm disturbances generated or made worse by anti-arrhythmic drugs. And they are quite common. In past decades, there have been fads for prescribing anti-arrhythmic drugs to asymptomatic people with ordinary ventricular ectopic beats (PVC's) and who have never had a heart attack. This is indefensible (Am. J. Card. 64: 50-J, 1989; NEJM 312: 193, 1985), and we can only guess how many thousands of people have died worldwide as a result.
|
|
|
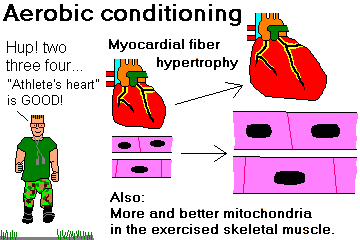
ATHLETE'S HEART (is good: Eur. Heart. J. 17: 127, 1996; making the call Prog. Card. Dis. 54: 387, 2012).
![]() Athletic heart
Athletic heart
Tom Demark's Site
The heart is special, because one of the most common "abnormalities" is the desirable result of vigorous aerobic training.
|
The athlete's heart is hypertrophied, often remarkably so.
Even supine, you can often feel the apex beat far lateral to
the mid-clavicular line. The pulse is slow (50-60 beats at rest), and the QRS complex often
tremendously large (why?).
* An athlete's hypertrophied ventricles also express atrial natriuretic factor, helping dispose of a sodium load -- perhaps this is why exercise helps patients with hypertension. * The right ventricle, of course, is often big as well (Prog. Card. Dis. 54: 397, 2012). Further, the athlete develops tremendous collateral circulation. If something happens to one coronary artery, the percentage of lost myocardium (if any) will be less. There will also be more total heart muscle remaining. If there is serious ischemia of a portion of heart, surviving a major rhythm disturbance may still be a problem, but death from cardiogenic shock in the acute phase is far less likely. |
 Maurice Greene comes from my home town |
Also, exercise does offer some protection from coronary artery atherosclerosis. Most runners also avoid tobacco and cholesterol-raising foods, and exercise tends to keep hypertension and adult-onset diabetes at bay, making it harder to sort out the benefit of exercise itself. Nevertheless, even the best runners enjoy no absolute immunity to coronary artery atherosclerosis, with all its serious side-effects.
People who weren't thinking (including some physicians in the 1950's) used to talk about "athlete's heart syndrome" as if it were something to be avoided. The "reasoning" was that failing hearts in disease tend to be hypertrophied, and.... Silly, okay. Probably the only common situation in which a person should avoid heavy aerobic training is some birth defects in which cardiac hypertrophy is likely to impair outflow from the left ventricle. (Outstanding among these is hypertrophic cardiomyopathy).
* One group reports that athletes' hearts do not hypertrophy to a thickness of more than 12 mm unless their chambers are also dilated, which should help make the distinction from diseased hearts: J. Am. Coll. Card. 40: 1431, 2002; this surprises me, as it does not square with my own experience after having autopsied a few high-performance athletes.
Your lecturer agrees with the position taken in Lancet 371: 1489, 2008 that neither routine ultrasonography nor routine EKG's for all athletes are worthwhile -- they will generate much confusion and expense and save very few lives.
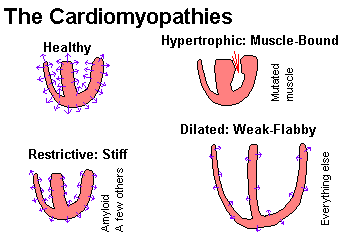
You'll need to know this somewhat artificial classification of cardiac hypertrophies, since a clinician or radiologist may ask you about it, and as there's talk about "remodelling of the heart" (NEJM 358: 1370, 2008), it may eventually come down to a real science.
ECCENTRIC HYPERTROPHY: The heart is big and it is NOT able to empty properly. The cardiac myocytes are LONGER, with new sarcomeres laid down BEYOND existing ones. Thick wall, chamber is very expanded and does not empty adequately. Perhaps the heart is pumping too much blood (anemia, AV shunts, thyroid disease, others) and/or it refills (aortic regurgitation) and/or there's a dead zone from an old healed infarct and/or it's doing its best but can't keep up for whatever reason (i.e., congestive heart failure from most causes). Looks bigger than a concentrically hypertrophied heart of the same weight on a chest x-ray. (Why?)
PHYSIOLOGIC HYPERTROPHY: Aerobic athlete; also late in pregnancy. Thick wall, the chamber can fill tremendously but empties very well. Word on the street is that hypertrophied cells are longer than they are thicker, but since so few specimens come in for study, the question's not settled. Watch for the molecular biology -- including more expressions of variant myosins -- to be uncovered as we learn more about conditioning.
HYPERTROPHIC CARDIOMYOPATHY: Uneven fiber enlargement and scrambling not to be confused with any of the above. Bumps on the heart muscle notably around the aortic outflow track.
This fascinating article also confirmed for me that no one knows the "normal weight" for the heart of an elite athlete / hard physical laborer, since so few die. For me, the question of when a medical examiner can say "the death was due to an enlarged heart" remains unanswered.
JAMA reports on deaths during triathlons (risk 1.5 per 100,000 per tri) confirms for me that "enlarged heart" in fit people without hypertrophic cardiomyopathy kills no one, though the authors reached a different conclusion. Of 14 death, 13 look like drownings, and one triathlete fell off the bicycle and broke his neck. Despite the drowning victims having "cardiovascular abnormalities" ("six had mild left ventricular hypertrophy".... they are triathletes, go figure), nobody dropped dead from anything resembling heart trouble during the running or biking. See JAMA 303: 1255, 2010.
Measurements for future pathologists:
350 gm... Traditional normal upper limit of weight for a (slim adult couch potato's) heart
1.5 cm... Traditional normal upper limit of thickness for a (slim adult couch potato's) left ventricle
0.5 cm... Traditional normal upper limit of thickness for a (slim adult couch potato's) right ventricle
Measurements don't include the trabeculae carnae.
Fun to know: If the heart was once very hypertrophic but is so no longer (i.e., an athlete gone to seed, a hypertensive or valve-disease patient successfully treated), the anterior and posterior descending coronaries are very wiggly "accordion arteries". Why? I've seen this often.
When someone dies suddenly with only a large heart -- concentric or eccentric hypertrophy, and the medical examiner finds no other cause of death, it's considered acceptable to blame a rhythm disturbance (NEJM 358: 1370, 2008 -- the article makes the point that in athletic/physiological hypertrophy, you do NOT get rhythm problems as a result). I've always been shy about doing this, but it comes up often in high-profile "sudden deaths of athletes." In my 1300+ autopsies, I have never had to invoke "enlarged heart" as cause of death.
CONGESTIVE HEART FAILURE ("CHF"; update Lancet 373: 941, 2009)
Inability of the heart to handle the volume of blood returned to it.
Either the heart muscle cannot pump because of intrinsic disease, or the blood is flowing in the wrong way, or the heart must pump against excessive resistance, or the heart must pump a preposterously large amount of blood (the latter is "high output failure").
Physiologists speak of "forward failure" (i.e., inability to perfuse the arteries, notably the kidneys to dispose of a sodium load) and "backward failure" (i.e., congestion and its problems). Both occur simultaneously, of course, but one or the other may be more obvious clinically.
The distinction between "congestive heart failure" and "cardiogenic shock" is admittedly artificial. "Cardiogenic shock" is a term reserved for the acute situation (usually a myocardial infarct); "failure" can simply mean inability to handle the ordinary venous return.
 As the heart is forced to work extra-hard, it undergoes HYPERTROPHY (i.e., more muscle
mass) and perhaps dilatation (i.e., chamber enlargement, which helps pump the blood; remember Starling's
Law?) Eventually, however, the heart's strength cannot increase further, and the organ appears to
give up (i.e., it stops "obeying Starling's Law"). Now the chamber does not
empty fully.
As the heart is forced to work extra-hard, it undergoes HYPERTROPHY (i.e., more muscle
mass) and perhaps dilatation (i.e., chamber enlargement, which helps pump the blood; remember Starling's
Law?) Eventually, however, the heart's strength cannot increase further, and the organ appears to
give up (i.e., it stops "obeying Starling's Law"). Now the chamber does not
empty fully.
Exactly why the over-burdened heart's strength starts to fail is often unclear, and its response to pharmacologic interventions often makes the picture more mysterious. There is talk of induction of an abnormal myosin isoenzyme which is a poor ATP-ase, decreased numbers of beta sympathetic receptors, etc.
There's much interest in the effects of heart failure itself on the heart, both changes in the shape of the heart that render its pumping and/or filling less effective ("remodelling"; Am. Heart J. 130: 153, 1995), and problems with the cells themselves (notably failure of reuptake of calcium from the sarcoplasmic reticulum; see Am. Heart J. 129: 684, 1995). The "Batista partial left ventriculectomy", in which some muscle is actually removed from the failing heart, is now common, though results are mixed. (Randas Batista was a heart surgeon in a tiny town in rural Brazil; his success in 1996 probably wasn't "ethical" by our standards, but the procedure's in use anyway.) The "dynamic cardiomyoplasty", in which muscle from the back is wrapped around the heart and given a pacer, and the metal mesh "heart sock" are both designed to help keep the ventricle shaped normally.
* I wasn't at all surprised to learn that in an 2008 autopsy series (Arch. Path. Lab. Med. 132: 1392, 2008), every one of 70 obese decedents had heart weight "above normal." Why is the heart of a fat person bigger? Is it simply from the extra exercise of carrying around 100-200 or more pounds of weight? ( think so -- "the simplext explanation is usually the best".) Or is it the result of lack of adiponectin secretion by overstuffed adipocytes (Nat. Med. 10: 1384, 2005)? How can anybody tell? Anyway, after bariatric surgery, at least in the young, the heart tends to return to normal size (J. Am. Coll. Card. 51: 1342, 2008).
You can help out a congestive-heart-failure person, somewhat, by improving aerobic muscle tone (i.e., more efficient burning of fuel), but exercise is no panacea (J. Am. Coll. Card. 25: 1239, 1995; J. Am. Coll. Card. 27: 140, 1996; JAMA 283: 3095, 2000).
* Future pathologists: Serum cardiac troponin T (late 20th century pre-hospital to screen for MI's: Am. Heart J. 138: 45, 1999) as a marker for how bad my congestive heart failure is today: Am. Heart J 138: 95, 1999.
Nesiritide, a natriuretic peptide originally found in brain, helps CHF: NEJM 343: 246, 2000; you can also measure levels to detect CHF (NEJM 347: 161, 2002); B-type natriuretic peptide triumphs as a way to distinguish CHF from other causes of dyspnea: NEJM 350: 647, 2004. No surprise.
* Watch galectin-3, both as a molecule produced by macrophages in the failing heart that causes the fibroblasts to make collagen, as a serum marker for severity of congestive heart failure, and as a target for therapy.
|
|
LEFT-SIDED CONGESTIVE HEART FAILURE
Failure of the left side of the heart to pump sufficient blood.
Except in the case of pure mitral stenosis (why?) or amyloidosis (why?), the left ventricle will be hypertrophied and dilated. The left atrium will usually be, also (and especially in mitral valve disease, why?)
THE COMMON CAUSES OF LEFT-SIDED FAILURE
Ischemia (old or recent myocardial infarct, ischemic muscle disease)
Aortic or mitral valve disease
Systemic hypertension
Myocardial disease / cardiomyopathy
NOTE: Of these, uncontrolled "systemic hypertension" (i.e., too much blood to push through too-narrow arterioles) is the most common; when the heart fails, blood pressure drops, making the true cause less obvious. See JAMA 273: 1363, 1996 (Framingham). How it progresses: JAMA 275: 1557, 1996; J. Am. Coll. Card. 25: 888, 1995.
THE COMMON EFFECTS OF LEFT-SIDED FAILURE
First, on exertion
Later, PAROXYSMAL NOCTURNAL DYSPNEA ("cardiac dyspnea"); on lying down for a while, fluid redistributes itself in the body, resulting in pulmonary edema. I think that the reason that it's paroxysmal (i.e., comes on all of a sudden) is that as the lungs become heavier (i.e., congestion, maybe edema) their weight presses on the pulmonary veins which in turn makes them more congested. Patients may throw the windows open at night, or learn to sleep on various numbers of pillows; you the physician will hear rales; the pathologist may see "brown induration" and hemosiderin-laden "heart failure" macrophages; remember these?
DIASTOLIC HEART FAILURE is a special situation in which the ejection fraction is normal but the person is still in failure. The ventricle will not relax / is too stiff to fill properly. Hence, diastolic volume is low. It is not rare; the pathophysiology is being worked out (NEJM 350: 1953, 2004). Death may result from a sudden gush of pulmonary edema fluid. Don't overdiagnose it... remember that in dilated cardiomyopathy, the overstretched wall of the heart may be stiff.
HIGH-OUTPUT FAILURE is a special situation in which the heart fails because the body demands more blood than it can pump. You'll see dependent edema probably because the veins of the body constrict extra-hard to return blood to the heart. The causes:
Anemia
Hyperthyroidism
High fever
Shunts between an artery and a vein
Beriberi (poor autonomic control)
Paget's disease of bone (abnormal bone vasculature)
Iatrogenic (i.e., shunts in dialysis)
RIGHT-SIDED CONGESTIVE HEART FAILURE
Failure of the right side of the heart to pump enough blood.
As you'd expect, the right ventricle and atrium will usually be hypertrophied and dilated.
THE COMMON CAUSES OF RIGHT-SIDED FAILURE
Pulmonary emboli (acute or chronic)
Any disease interfering seriously with lung ventilation
Emphysema
Cystic fibrosis
Fibrosing lung
Most others
NOTE: The mechanism, of course, is increased pulmonary vascular resistance (due to fibrosis and/or the hypoxic vascular response; remember this?)
Left-sided heart failure!
Cardiac defects with left-to-right shunts (why?)
THE EFFECTS OF RIGHT-SIDED FAILURE
Splanchnic congestion (you'll feel big livers & spleens; check for "hepatojugular reflux")
Jugular venous distention (look carefully)
Edema in the feet and elsewhere (from increased venous hydrostatic pressure, etc.)
Effusions (transudates, of course; notably pleural, notably more on the right side than on the left; why?)
NOTE: "Cardiac cirrhosis" of the liver, often discussed in textbooks as the result of right-sided failure, almost never happens. The one time you might see it is in longstanding, severe tricuspid insufficiency, with or without right-sided failure (why?) If you are this sick, "cardiac cirrhosis" is probably the least of your worries.
NOTE: Some pathophysiologists include cardiac tamponade as a type of right-sided failure.
* Good news: In contrast to studies of selected patient populations with various illnesses from decades ago, black and white people with congestive heart failure seem to get equally good treatment (JAMA 289: 2517, 2003). This seems to be part of a general trend to eliminate (and even reverse) the past tendency to undertreat minority patients (NEJM 354: 1147, 2006).
NOTE: Especially with new biotech products, watch for more aggressive treatment of anemia of chronic disease as a way of helping your CHF patients (Am. Heart. J. 155: 751, 2008).
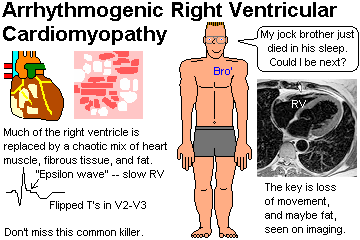
ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY ("arrhythmogenic right ventricular dysplasia"; Uhl's; "parchment right ventricle"; J. For. Sci. 42: 32, 1997; Circulation 94: 983, 1996; Arch. Path. Lab. Med. 129: 1330, 2005; South. Med. J. 101: 309, 2008; Am. Fam. Phys. 73: 1391, 2006; Lancet 373: 1289, 2009; sudden death AMFJP 30: 78, 2009 progressive nature of the lesion with apoptosis Am. J. Path. 152: 479, 1998; sudden death in vigorous good health AJFMP 18: 345, 1997).
The pathologist sees a curious mix of fatty ingrowth and fibrosis in the wall of the right ventricle (sometimes the left also or instead). We're working out the pathology and mechanism. The cell pathology is bizarre (Am. J. Med. Sci. 320: 310, 2000). First series of 200 deaths: Circulation 108: 3000, 2003.
Today's diagnostic imaging specialist must be familiar with this entity. You'll follow-up suspicious EKG"s with MRI's, which show both the fat (less important, since it's often mostly fibrosis instead of fat) and the dysfunction (more important). Update Am. Heart. J. 155: 147, 2008.
|
|
|
|
|
|
|
|
|
|
DILATED ("flabby heart"; "congestive cardiomyopathy")
{03629} dilated cardiomyopathy vs. normal
{03632} dilated cardiomyopathy
{07769} dilated cardiomyopathy
{11555} dilated cardiomyopathy
![]() Dilated Cardiomyopathy
Dilated Cardiomyopathy
CDC
Wikimedia Commons
There are a host of lesions that cause the heart muscle to lose its strength. There's a list in "Big Robbins"; most cases never get a diagnosis (NEJM 331: 1564, 1994; still probably true). Only 50% of cases of dilated cardiomyopathy even have a diagnosable lesion on microscopy.
In the dilated cardiomyopathies, the heart usually weighs more than "normal", but it is very distended and thus the wall is usually of normal thickness or even unusually thin. (Why?)
ALCOHOLIC'S HEART
Alcohol is rough on most organs, and the liver and brain damage take precedence most of the time to the cardiac toxicity.
Alcohol may or may not have a direct toxic effect on the heart, by itself or through a metabolite.
Lots of alcoholics are deficient in vitamin B1, and this can produce high-output failure or even weaken the muscle.
In 1966, some folks added cobalt to Quebec beer to make a nicer head of foam. Cobalt poisons pyruvate metabolism and produced a beriberi-like cardiomyopathy. (* The classic pathology: Ann. N.Y.Acad. Sci. 156: 577, 1969).
Selenium deficiency led to an epidemic of dilated cardiomyopathy in China, and occurs in patients in the developed world who suffer from malabsorption (Med. Sci. Law 42: 10, 2002).
PREGNANCY
Around the time of parturition and/or up to six months after, some women get a dilated cardiomyopathy. This "peripartum cardiomyopathy" is very serious: JAMA 283: 1183, 2000 (NIH conference).
Sadly, this is mostly a disease of poor women, especially those who have had several babies close to one another. Perhaps it is the result of a deficiency in some trace nutrient. It remains mysterious: Am. Heart J. 130: 860, 1995; and it's likely to recur: NEJM 344: 1567, 2001.
* Today, some people believe prolactin inhibition should be used as part of the treatment (Ann. Thor. Surg. 91: 274, 2011). There are a variety of empirical treatments, the disease can hang around for a long time, and several percent of women still die of it
ACROMEGALY (apoptosis of myocytes for some reason): Circulation 99: 426, 1999.
POLYMYOSITIS-DERMATOMYOSITIS can produce a cardiomyopathy, accelerate atherosclerosis, and/or promote widespread small-artery disease, depending on the patient (Rheumatology 45 S4: iv18, 2006.)
GENETIC SYNDROMES: Syndromes worth remembering for general medicine include Friedreich's ataxia and Duchenne's-Becker's muscular dystrophy. Even female carriers of the latter often get a dilated cardiomyopathy (JAMA 275: 1335, 1996).
Lately we've noticed that around 15% of idiopathic dilated cardiomyopathy patients have a mutation in one of the genes that produce desmosomes (Heart 97: 1744, 2011). Stay tuned.
There are some other syndromes that give only a dilated cardiomyopathy (* one is due to mutant actin: Circulation 99: 1022, 1999; others to mutant troponin T (pathology Circulation 104: 1380, 2001), and beta-myosin heavy chain NEJM 343: 1688, 2000; mutated desmin affects skeletal and cardiac muscle NEJM 342: 770, 2000; phospholamban Science 299: 1410, 2003; troponin I: Lancet 363: 371, 2004 is unusual for being recessive). Nexilin, a Z-band protein: Nat. Med. 15: 1281, 2009.
"Noncompaction cardiomyopathy" ("spongiform cardiomyopathy"; Circulation 108: 2672, 2003) features very much enlarged trabeculae with blood lakes in the wall or one or both ventricles. Easy to spot on echo (Am. J. Card. 94: 389, 2004), it is is acquired during embryogenesis, either with a known mutation / syndrome or by itself. It can produce a dilated or a restrictive pattern.
Remember sarcoidosis, Chagas, radiation, giant-cell myocarditis, rheumatic fever, ipecac (Munchausen's by proxy: Pediatrics 97: 902, 1995).
"Stress cardiomyopathy / myocardial stunning / transient apical ballooning syndrome / broken heart syndrome", after a jolt of epinephrine, has been precipitated even by surprise parties (NEJM 352: 539, 2005) or opiate withdrawal (Mayo Clin. Proc. 81: 825, 2006). The quaint term for this illness, which features ballooning of the ventricle especially at the apex, raised cardiac enzymes, and generally a good outcome, is "takotsubo", Japanese for "octopus trap". There's one report of rupture after a quarrel (literally dying of a broken heart: Mayo Clin Proc. 79: 821, 2004). Update JAMA 306: 277, 2011.
Seldom fatal, but worth knowing: In the most severely injured patients, notably those with SEVERE BURNS, there is weakening of the heart; in the animal model, there is widespread apoptosis of cardiac myocytes (J. Trauma 65: 401, 2008).
Seldom fatal, but worth knowing: A common cardiac lesion in patients with chronic renal failure (UREMIC CARDIOMYOPATHY) is fibrosis around individual fibers throughout large areas. This greatly enlarges the left ventricle, but doesn't really interfere so much with function at least during systole. Update Kid. Int. 69: 1839, 2006. People who've been on hemodialysis for a long time often have calcium chunks in the heart; these can be huge but seldom cause problems (Medicine 91: 165, 2012).
* The old story about the earlobe crease as a marker for precocious atherosclerosis holds up on the Swedish autopsy service: AJFMP 27: 129, 2006.
* Still controversial is whether diabetes itself causes a cardiomyopathy, perhaps by increasing the connective tissue among the myocardial cells. See Heart 92: 296, 2006; J. Am. Coll. Card. 47: 693, 2006).
* The left-ventricle assist device (plus a medical regimen) sustains patients with cardiomyopathy as it heals over time. This now seems to be a life-saver, and will probably be fairly common when you are in residency. See NEJM 355: 1873 & 1922, 2006.
HYPERTROPHIC ("idiopathic septal hypertrophy", "asymmetric septal hypertrophy"; "Les Aspin's disease"; "Reggie Lewis's disease"; "muscle-bound heart"; Hank Gathers supposedly had myocarditis rather than hypertrophic cardiomyopathy NEJM 329: 55, 1993)
|
{07811} hypertrophic cardiomyopathy
|
This curious lesion, generally autosomal-dominant, exhibits (1) variable regions of hypertrophy (in contrast to the "concentric hypertrophy" of left ventricular overwork and the "eccentric hypertrophy" of left ventricular overfilling); (2) disarray of cardiac muscle cells by light and electron microscopy; (3) fibrosis around muscle cells; (4) some fibrous narrowings of the small arteries.
This is serious. The outflow tract from the left ventricle toward the aortic valve is likely to be obstructed by the large mass of muscle ("obstructive hypertrophic cardiomyopathy"; "HOCM").
Be careful! If you do aerobic exercise and develop your heart muscle, you're likely to make this particular problem worse. (Here's the case for routine auscultation of the heart in would-be athletes. See J. Fam. Pract. 52: 127, 2003 for the pre-sports physical, author recommends what I've always done and taught.) The septum typically takes its thick shape in the teenaged years. Like other causes of anatomic or functional aortic stenosis, patients are troubled with exercise intolerance, angina, syncope, and/or sudden death. Infamously, sudden death can be the first "warning".
Most cases are autosomal-dominant, and a majority are caused by a mutated beta-myosin gene. Mouse with the mutation: Science 271: 663, 1996. Other loci are now known, mutant troponin T is common and especially deadly (histopathology Circulation 104: 1380, 2001), and tropomyosin and myosin binding protein are rare (NEJM 332: 1058, 1995; J. Am. Coll. Card. 29: 635, 1997; NEJM 338: 1249, 1998; Lancet 355: 58, 2000).
* Danon's cardiomyopathy (gene LAMP2; JAMA 301: 1253, 2009 -- one of those rare X-linked semi-dominant diseases), a systemic disease with glycogen storage vacuoles, can produce an extreme hypertrophic cardiomyopathy, with or without an extra-thick septum / crisscross fibers.
Many patients die suddenly in youth. But many others do not die of the disease, or die of it in old age (CHF or rhythm problems: JAMA 281: 650, 1999).
* When in doubt about how to manage this, put the patient on a treadmill. Dropping the blood pressure is a warning of more ominous things to come. The risk for sudden death cannot be predicted simply by measuring the thickness of the wall (Lancet 357: 420, 2001). Gadolinium scan to predict risk: J. Am. Coll. Card. 41: 1561, 2003.
The implantable defibrillator for these people: NEJM 342: 422, 2000.
Today, alcohol ablation of the obstructing muscle mass (i.e., alcohol injected into the coronary arteries) is a well-established therapy for patients not able to tolerate the surgery. Pathologists see Am. J. Card. 99: 563, 2007 and especially Heart 92: 1773, 2006 (coagulation necrosis of the coronary arteries themselves). As you'd expect, heart block may be unavoidable.
* Forme frustes? There are several genes (sarcomeres, mitochondria) which produce rare genetic cardiomyopathies. In the Framingham study, about person in 30 has unexplained, mild left ventricular hypertrophy and of these, one in 5 is heterozygous for one of these (Circ. 113: 2697, 2006); we await full data on the prevalence of these mutations in those without hypertrophy, but this is worth watching.
* The name of Reggie Lewis will always be associated with hypertrophic cardiomyopathy. However, this was not found on ultrasonography in life, or at autopsy. The extensive scarring described at autopsy sounds much more like "cocaine heart". During the litigation, which finally ended in 2005, there was a great deal of lawyering by attorneys for his family about Lewis's failing and/or refusing to take drug tests for cocaine. Of course, we'll never know for certain.

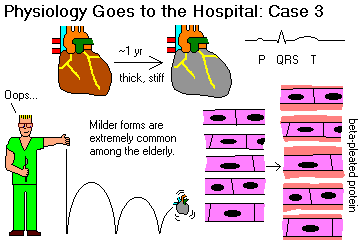
RESTRICTIVE-INFILTRATIVE-OBLITERATIVE ("stiff heart"; usually amyloid)
Most older people get some amyloids of various compositions (known and unknown) in their atria and aortas. All about amyloid and the heart: J. Clin. Path. 58: 125, 2005; clinicians see Am. J. Med. 124: 1006, 2011.
If amyloid involves the myocardium extensively, the muscles cannot contract. This is the usual cause of "restrictive cardiomyopathy" in the developed world. Cardiac amyloidois Am. J. Med. 124: 1006, 2012.
* Amyloid hearts bounce; I was shown this once. Don't try it; it's disrespectful.
* Fabry's storage disease produces a restrictive cardiomyopathy, and sometimes a very severe case of sarcoidosis or hemochromatosis can do the same.
* A small minority of hypertrophic cardiomyopathy patients (with the same genes as the classic presentation) have a stiff heart with little hypertrophy (J. Am.Coll. Card. 49: 2419, 2007); the prognosis is of course worse than in the classic syndrome.
Some "restrictive cardiomyopathy" patients, both children and adults, have developed an idiopathic interstitial fibrosis (i.e., collagen rather than amyloid) that makes the heart stiffer.
We've already seen endocardial fibroelastosis as a mimic, and some of the diseases of skeletal muscle can also produce a restrictive cardiomyopathy (Heart & Lung 40: e123, 2011).
{07937} amyloid heart
{07940} amyloid heart
{07943} amyloid heart
SECONDARY CARDIOMYOPATHIES: Hemochromatosis (dysrhythmogenic!), pheochromocytoma ("catecholamine heart", with single-fiber necrosis/apoptosis as in cocaine heart and perhaps after burns), chemotherapy (doxo- and daunorubicin; "Adriamycin"; flabby dilated heart), Pompe's glycogenosis, other glycogen storage diseases (glycogen synthetase deficiency NEJM 357: 1507, 2007), Duchenne's (dilated cardiomyopathy), Friedreich's (a variety of phenotypes Circulation 125: 1626, 2012; as a mimic for hypertrophic cardiomyopathy -- Irish J. Med. Sci. 180: 799, 2011), end-stage HIV infection (Br. Med. J. 309: 1605, 1994).
Again, Fabry's is worth knowing, because it can mimic hypertrophic or restrictive cardiomyopathy, and is treatable with enzyme replacement (Circulation 105: 1407, 2002). Around 0.5% of "hypertrophic cardiomyopathy" patients turn out to have Fabry's (Heart 97: 1957, 2011).
Adriamycin is hated for producing a characteristic dilated cardiomyopathy. You may see pale-blue-staining myocardial cells without sarcomeres ("adria cells") next to normal cells. Adriamycin damages the mitochondria and somehow complexes with iron, producing free radical injury to the surrounding sarcomeres (J. Clin. Inv. 117: 3730, 2007). Anyone treated with adriamycin is likely to have fibrosis between the myocardial cells for the rest of their lives -- future pathologists be aware of this.
I'm not big on the term ISCHEMIC CARDIOMYOPATHY for the fibrosis of the heart that's sustained many little infarcts, since the disease is not primarily the muscle. It's come into general use, though, and it's diagnosable on imaging -- remember that it remodels (Am. J. Card. 109: 390, 2012).
{07039} von Gierke's disease
{07958} hemochromatosis
{07967} Fabry's
{08053} pheochromocytoma heart
{08078} radiation atherosclerosis
{08081} radiation pericarditis
* CADAVERIC SPASM
This is instantaneous rigor mortis, generally seen in extreme pain or fright. It's said that the heart can stop as well in this setting. The mechanism must be influx of calcium into skeletal and cardiac muscle, as in rigor mortis.
There's no sorting this out, or saying whether this is "on a continuum with stunned myocardium."
This is especially helpful in drownings, where water weeds held tight in the hands are proof that the person was alive in the water.
There are old reports from the battlefield of soldiers found dead, or seen to die, with no injury, but with their muscles frozen in position, generally after a shell had exploded nearby. The cause was supposedly terror. Since the accounts are of groups of soldiers all dying at once, your lecturer believes these were blast injuries, with internal damage not visible on the outside, and that the bodies were found after rigor had developed normally.
If cadaveric spasm really occurs, it must be rare. It makes for good stories, though. It's also the origin of the comforting untruth that, if you fall or jump off a building or something and see that you will surely die, your heart will stop before you hit the ground.
PERICARDIAL DISEASE (Lancet 363: 717, 2004)
|
|
PERICARDIAL EFFUSIONS
Many different situations result in accumulation of fluid (i.e., more than the usually few mL) in the
pericardial sac. Viral pericarditis is often accompanied by a serous effusion; TB's![]() effusion may also
be serous.
effusion may also
be serous.
However, to produce tamponade, it takes around 150 mL of fluid, and it must build up pressure rapidly (i.e., hemopericardium after ventricular rupture, penetrating trauma, or aortic dissection; pus in a suppurative pericarditis). If the fluid accumulates slowly, the pericardium can expand. I've seen a one-quart effusion that didn't seem to cause a problem.
* "Recurrent pericarditis" is a nuisance illness that seems to do little harm; your lecturer had long believed that it is a variant of familial mediterranean fever and this has been borne out now by the striking success of colchicine to end attacks (Am. Fam. Phys. 85: 1098, 2012).
On rotations, you'll learn how to do pericardiocentesis, and to place a pericardial window to keep the fluid "off the heart".
PERICARDITIS
Inflammation of the pericardium hurts. Patients typically get relief by leaning forward. You, the physician, will strain to hear the notoriously ephemeral "friction rubs" of this illness.
In sporadic pericarditis, the etiologic agent is probably some virus or immune phenomenon. It usually is self-limited; today people treat it with NSAIDs.
"Fibrinous pericarditis" (the familiar "bread and butter", or more realistically, "ketchup on bread" lesion) results from myocardial infarction (over a transmural infarct; Dressler's), uremia, radiation, lupus, Behcet's (surprisingly common Medicine 91: 25, 2012), rheumatic fever, surgery, and trauma. It may organize (obliterating the pericardial space, which isn't usually a big deal), or even calcify (perhaps limiting distensibility).
{18719} fibrinous pericarditis
|
|
|
Pus in the pericardial space usually means bacterial infection. They usually got there by way of a
nearby pneumonia or empyema, though there are other routes. Again, it may organize (a few
adhesions, or "obliterative pericarditis"; or even calcify. TB![]() in particular is infamous for causing
troublesome scars. Old healed pericarditis (or TB, or asbestos, or amyloid
selectively involving the pericardium, or "idiopathic")
may become "constrictive" and cause problems.
in particular is infamous for causing
troublesome scars. Old healed pericarditis (or TB, or asbestos, or amyloid
selectively involving the pericardium, or "idiopathic")
may become "constrictive" and cause problems.
![]() Suppurative pericarditis
Suppurative pericarditis
WebPath photo
"Hemorrhagic pericarditis" is due almost exclusively to TB![]() and cancer.
Caseous
and cancer.
Caseous![]() material in the
pericardial sac is probably TB.
material in the
pericardial sac is probably TB.
{03659} constrictive non-calcific pericarditis
* The "soldier's plaque" or "milk spot" on the visceral pericardium (i.e., the epicardial surface) is a focus of very slight thickening of the fibrous layer under the mesothelium. This mysterious finding is harmless. It was once attributed to packs worn during WWI, until some clever person noted that it's equally common in women. Today, somebody may tell you it represents "healed pericarditis", but it doesn't really look like scar.
CARDIAC TUMORS are rare.
The principal primary heart tumor is the MYXOMA, a lesion of endothelial origin that arises as a ball from the atrial septum and fills the left atrium. Grossly, it's a typically benign, soft tumor. Microscopically, there are lots of spindle cells and ground substance, and few vessels. Myxomas (familiarly called "wrecking balls") can plug the mitral valve (instant death), or cause emboli (under-recognized, Chest 107: 674, 1995).
Atrial myxoma are really tumors, not just organized atrial "ball-valve" thrombi of the sort seen in mitral valvular disease. They're clonal, and have a few antigenic markers.
* Being genuine tumors, there's an important anti-oncogene deletion syndrome ("Carney complex", with lentigos and gland overfunctions too, and others: Am. J. Card. 79: 994, 1997). If you see a myxoma in the right atrium rather than the left, suspect a familial syndrome.
![]() Atrial myxoma
Atrial myxoma
AFIP
Wikimedia Commons
People with tuberous sclerosis are prone to have small cardiac hamartomas ("rhabdomyomas"). These are seldom troublesome.
* The other pediatric tumor of the heart is the troublesome cardiac fibroma, which juts off the septum and interferes with outflow. Thankfully, it's rare.
I was amazed by my first PAPILLARY FIBROELASTOMA (J. Thor. Card. Surg. 112: 551, 1996; fatal AJFMP 19: 162, 1998) at a 1991 autopsy. It looked like a kooshball on the pulmonic valve. These form on any valve leaflet. Depending on location, they can cause embolic stoke (Clin. Card. 24: 346, 2001); look for them using transesophageal echocardiography. Series Circulation 103: 2687, 2001; surgery Ann. Thor. Surg. 80: 1712, 2005. Smaller, similar-looking lesions on the aortic leaflets called LAMBL'S EXCRESCENCES are supposed to be organized thrombi; they occasionally embolize too.
Lipomas ("lipomatous hypertrophy" / "massive fatty deposits") of the septum are occasionally seen (review AJFMP 17: 43, 1996); if it interferes with flow, it can be removed.
* Intracardial leiomyomatosis is the presence in a cardiac chamber of a leiomyoma that has extended directly from a vein -- it is common enough to have a surgical literature (J. Thor. Card. Surg. 142: 823, 2011).
* "Cystic tumor of the AV node" is a hamartoma that mimics the ultimobranchial nests of the thyroid (Am. J. Clin. Path. 123: 369, 2005).
{08029} lipomatous hypertrophy ("lipoma") of atrial septum
* The AV node is subject to a poorly-understood, rare lesion called "cystic tumor" (formerly "mesothelioma", a misnomer). Epithelially-lined cysts are filled with mucin. Heart block can result.
Primary cancers of the heart (i.e., sarcomas) are so rare that even Mayo's sees only about one a year; of course, they are deadly (Cancer 112: 2440, 2008.)
Metastases to the heart are usually from lung, breast, melanoma, lymphoma, leukemia, and choriocarcinoma. Any of these can be troublesome (heart block, muscle failure, effusion), but you will usually miss the diagnosis during life.
LAST PHOTOS
{17416} tiger-stripes of fatty change in extreme anemia; remember this one?
{11480} thromboembolus caught in heart

PULSUS PARADOXUS OVERSIMPLIFIED
You need to understand this. In health, venous pressure drops slightly during inspiration because the decreased intrathoracic pressure draws venous blood into the chest.
In health, arterial pressure drops slightly during inspiration because the greatly expanded pulmonary vascular bed takes up the right cardiac output, diminishing return to and output from the left. The resulting systemic arterial pressure drop is less than 10 torr and is not obvious on palpation.
In restrictive pericardial disease, the diaphragm pulling down on the pericardium pulls it even tighter. This further blocks venous return to the heart, so that venous pressure actually increases during inspiration. The neck veins are more distended during inspiration, and drop during expiration. In restrictive pericardial disease, breathing in also blocks arterial outflow from the heart, greatly accentuating the normal drop in arterial pressure during inspiration (>10 torr, should be detectable on palpation).
|
FINISHING UP
* The desires of the heart are as crooked as corkscrews.
--W.H. Auden
*Transgenic pigs are now being bred as heart donors: Br. Med. J. 312: 657, 1996. Since then, there has been a great deal of work, but with publications only in the super-specialty literature. Stay tuned.
Cats look down on us. Dogs look up to us. Pigs treat us as equals.
-- Winston Churchill
You'll hear in ACLS about ELECTROMECHANICAL DISSOCIATION, i.e., the EKG is fine and the heart isn't pumping. In other words, dead with a normal EKG. What would cause this? HINT: Pulmonary emboli, tamponade, no venous return to the heart because of bleeding or widespread vasodilatation, ruptured septum, no calcium. Understand the "why" of each? Can you think of others? Since the only one that you're going to be able to treat during CPR is "no calcium", you'll give calcium by vein. Don't expect....
In our sleep, pain which cannot forget falls drop by drop upon the heart until, in our own despair, against our will, comes wisdom through the awful grace of God.
-- Aeschylus, "Agamemnon"
tr. by Robert F. Kennedy in his extremporaneous eulogy for Martin Luther King
* A final, personal note: The heart is a source for metaphor. My favorite quotations concerning the heart include Jeremiah 17: 9-10 and 31:33 and Koran 91:4. See also the Katha Upanishad for a classic description of the metaphysical heart. And of all the experiences in clinical medicine, the one that this pathologist loves the most is the sound of the beating heart.