![]() Adrenal gland
Adrenal gland
"Pathology Outlines"
Nat Pernick MD, great site
Title: Tubulointerstitial Disease
Date & Time: Wednesday, January 22, 2014 at 8 AM
Lecturer: The Pathology Team
QUIZBANK
Endocrine (It's impossible to separate pituitary, adrenal, thyroid, parathyroid, etc. Look at it all now.)
MAINTAIN A HIGH INDEX OF SUSPICION FOR ENDOCRINE DISEASE. This lecture ought to scare you.
|
|
|
One surprising fact about the adrenal gland is that, unlike many other organs, masses found here are seldom biopsied prior to excision. The radiology team will advise surgeons whether to remove particular masses. The one exception is biopsy to confirm metastatic disease in someone with a known cancer, usually in the lung (Arch. Surg. 144: 465, 2009).
THE ADRENAL CORTEX: "An organ essential to life." Pathology of the adrenal cortex: Arch. Path. Lab. Med. 132: 1263, 2008.
|
{11204} adrenal and its nerve, normal
{11207} adrenal and its nerve, normal {11210} adrenal and its nerve, histology, normal {15035} normal adrenal gland, showing zones (can you figure them out?) |
|
Unlike the pancreas, it isn't obvious where the "head", "body", and "tail" of an adrenal is located. The head is medial, the tail lateral.
The cortex, of course, has three "zones":
(1) ZONA GLOMERULOSA: mineralocorticoid production. Thin and patchy, small cells. (Most sources today say that ACTH does not affect the zona glomerulosa or mineralocorticoid production.)
(2) ZONA FASCICULATA: glucocorticoid production (now seems settled), resting cells (the reserve cells are at the ZG-ZR interface). Yellow.
(3) ZONA RETICULARIS: glucocorticoid production, androgen and estrogen production, grossly darker than outer layers. Brown.
(Mnemonic: Salt, sugar, and sex: the deeper you go, the sweeter it gets.)
Under the microscope, the borders are fairly easy to see.
In newborns, the future adult cortex is a thin layer under the capsule, and most of the gland is "fetal zone". This regresses in a few weeks and is usually gone altogether by the first birthday.
Future pathologists:
The normal adult weight of each adrenal gland is 4 gm.
If an adrenal gland weighs 6 gm or more (without a tumor), it is usually hyperplastic. The stress of the final illness increases the weight of the adrenals, which is why "normal autopsy weight" of an adrenal is sometimes given at 6-8 gm. Most violent suicides have adrenals weighing 9-11 gm each (Am. J. Psych. 144: 1214, 1987; confirmed AJFMP 19: 72, 1998.) The fetal adrenal, if enlarged, warns of impending premature labor.
* More for future pathologists: A bit of ectopic marrow, a few pigmented cells, or theca cells (especially in menopausal women) are normal.
* Ask a physiologist about the role of dehydroepiandrosterone in health; about 30% of a man's androgens are derived from this, and 90% of a post-menopausal woman's estrogens.
{49431} hyperplasia of adrenal cortex, etiology undisclosed
{09217} adrenal cortical hyperplasia, etiology unknown
* Around 2% of folks have adrenals with black nodules, usually without any evidence of dysfunction.
CONGENITAL ADRENAL HYPOPLASIA
Two types, both uncommon:
(1) Anencephalic type: thin cortex, no fetal zone; no ACTH during development
(2) Cytomegalic type: thin cortex composed entirely of large, bizarre cells (foci of such cells are common in normal newborns but regress).
It now turns out that a forme fruste allele at the DAX1 locus causes adrenal insufficiency in boys (no adults yet), i.e., this is a common, previously-unrecognized cause of adrenal insufficiency (J. Clin. Endo. Metab. 91: 3048, 2006).
Both present as hypoglycemic seizures in infants. Glucocorticoid replacement saves these children's lives.
ECTOPIC ADRENAL CORTICAL TISSUE (sometimes ectopic adrenal medulla too)
This is most common in the capsule ("capsular extensions") and at the origin of the celiac artery, but it can occur anywhere in the retroperitoneum, or under capsules of liver, kidney, ovary, or testis.
Ectopic adrenal caused problems when surgical adrenalectomy was very popular. ("A new adrenal gland grew back in a different place....")
| HYPOADRENOCORTICISM ("Addisonism", etc.): Insufficient glucocorticoid (and usually insufficient mineralocorticoid) production. Reviews Lancet 361: 1881, 2003; NEJM 360: 2328, 2009. |
|
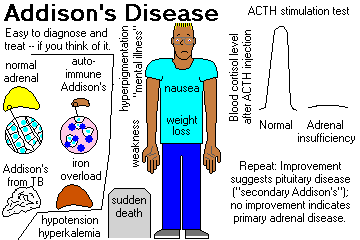
CHRONIC HYPOADRENOCORTICISM (ADDISON'S DISEASE, now regardless of etiology). Troubles start when 80% of the gland tissue is gone. Review Br. Med. J. 312: 1085, 1996.
{09223} adrenal tuberculosis, gross
{25399} tuberculosis of adrenal, histology
{27257} tuberculosis of adrenal, histology
|
![]() TB of the adrenal
TB of the adrenal
WebPath Photo
Worldwide, fungal infections (remember histoplasmosis![]() ,
coccidioidomycosis
,
coccidioidomycosis![]() and South American blastomycosis)
and leprosy
and South American blastomycosis)
and leprosy![]() are important
causes, and now AIDS is too.
are important
causes, and now AIDS is too.
AUTOIMMUNE
The most prevalent non-iatrogenic cause of Addison's disease in the US today. The adrenal remnants are typically loaded with lymphocytes, etc., etc. Jack Kennedy suffered from this illness in his youth, and it was missed for several years (JAMA 201: 115, 1990). Even today, "delayed diagnosis of adrenal insufficiency is common": Am J. Med. Sci. 339: 525, 2010 ("twenty percent suffered for more than five years" -- especially when the principal complaint is "just not feeling well" / "low subjective health status").
![]() Jack Kennedy, second from left
Jack Kennedy, second from left
PT109 reunion, 1944
Notice weight and pigmentation
Long-mysterious, it's now clear that most of these patients have autoantibodies against 21-hydroxylase (Lancet 339: 1559, 1992). These are now generally called "adrenal cortex antibodies." Presumably this is antibody-dependent cell-mediated cytotoxicity, as in Hashimoto's disease.
And around 1.5% of people with type I diabetes make these antibodies, with progression to disease determined by genetic factors (J. Clin. Endo. Metab. 97: 1573, 2012).
Autoimmune adrenalitis often occurs jointly with Hashimoto's thyroiditis, type I diabetes mellitus, vitiligo ("autoimmune polyendocrine deficiency syndrome II", "polyglandular failure type II"; "Schmidt's syndrome", etc.)
Schmidt's is only the best-known of the autoimmune polyendocrinopathies in which there are various immune and non-immune disturbances tending to run together.
* There are probably other multi-antuoimmune diseases that are being worked out; consider endocrine disease when a patient with vitiligo or prenicious anemia seems unwell.
The more common Schmidt's (autoimmune polyglandular syndrome type II) is officially diagnosed when there is autoimmune addisonism plus either autoimmune thyroid disease or autoimmune diabetes (Am. Fam. Phys. 75: 667, 2007).
* Autoimmune polyglandular syndrome type III is diagnosed when there is autoimmune thyroid disease plus one or more other autoimmune diseases other than addisonism or hypoparathyroidism (Am. J. Med. Sci. 333: 178, 2007; J. Clin. Endo. Metab. 97: E1043, 2012). This is likely to change as these illnesses are further defined by molecular biology.
IATROGENIC
This results from too-rapid withdrawal of glucocorticoid medication, post-adrenalectomy for breast cancer or Cushingism, etc., ketoconazole or fluconazole antifungal drug therapy (Crit. Care Med. 29: 668, 2001), removal of a "non-functioning adenoma" (rare).
CORTICOSTEROID INSUFFICIENCY OF CRITICAL ILLNESS is a newly-characterized entity, seen especially in severe systemic infections, in which the body does not put out enough glucorticoid to handle the extra stress. The pathology's not worked out yet, but the impact on clinical practice in the ICU will be huge, since one must know whether to give supplementary cortisol. Consensus statements from the American College of Critical Care Medicine: Crit. Care Med. 36: 1937, 2009. See also Chest 135: 181, 2009.
OTHERS: Worth remembering are
{25394} adrenal cortical atrophy (key says "hypoplasia", I doubt this)
{24607} adrenal amyloidosis, gross
{15960} cytomegalic inclusion disease![]() , adrenal
, adrenal
{37216} adrenal leukodystrophy ("Lorenzo's oil") case, gross brain
{37218} adrenal leukodystrophy case, gross brain
{37221} adrenal leukodystrophy case, histology brain
{37224} adrenal leukodystrophy case, gross adrenal
{37225} adrenal leukodystrophy case, histology adrenal
* NOTE: Hollywood is Hollywood. The aftermath of "Lorenzo's oil" was disillusionment. It failed controlled studies miserably (NEJM 329: 745 & 801, 1993; NEJM 330: 1904, 1994; Ann. Neuro. 34: 121 & 169, 1993), and poisoned platelets (NEJM 328: 1126, 1993; Am. J. Hem. 44: 290, 1993; J. Inh. Metab. Dis. 17: 628, 1995) and (at least sometimes) natural-killer lymphocytes (J. Inh. Metab. Dis. 18: 101, 1995). We've now got two series of dead adrenoleukodystrophy patients who were treated in life with Lorenzo's oil. The scientific community pointed out that "Lorenzo's oil" doesn't even cross the blood-brain barrier, which is probably why it didn't work (Neuroch. Res. 19: 1073, 1995; Ann. Neuro. 36: 741, 1995). Yet another massive failure: J. Neurol. Neurosurg. Psych. 67: 290, 1999. However, interest continued. A study from Hopkins (Arch. Neuro. 62: 1073, 2005) that claimed success in preventing lesions in asymptomatic boys had only historical controls and also included other dietary alterations. Since the disease has a variable course in members of the same family, it's impossible to tell how much of the benefit was related to the treatment.
ACTH DEFICIENCY ("secondary hypoadrenocorticism")
These patients have almost always lost their adenohypophysis and have "panhypopituitarism". (Treat the whole person.... Caring for a little pituitary dwarf? Don't get focused on the height so that you forget the likely adrenal insufficiency.... J. Clin. End. Metab. 81: 1693, 1996). Less often, they have selective, presumably autoimmune, loss of the ACTH-producing cells: Arch. Int. Med. 152: 1705, 1992.
Clinical picture:
"Addisonian" patients show weakness, nausea, and weight loss, and are usually hypotensive (* 110/70 or less) and have other complaints. Like most endocrine patients, the problems are likely to appear "emotional".
In primary hypoadrenocorticism, the skin and buccal mucosa will usually be hyperpigmented, due to increased ACTH (MSH?) -- also look at freckles, nipples, palmar creases, old scars.
Lab studies typically show hyponatremia, hyperkalemia, metabolic acidosis, hypoglycemia, low serum cortisol, low urinary 17-OH-steroids, and (most important) failure to respond to various "stimulation tests" by increasing cortisol output.
It is common for these patients to die suddenly and unexpectedly before anyone thinks of adrenocortical insufficiency. This still happens (Br. Med. J. 312: 1085, 1996).
* Osteoporosis is severe in post-menopausal women with Addisonism, because of loss of adrenal androgens.
Replacement therapy is life-saving. (And get your patient a syringe of cortisol and an ID bracelet.)
{09371} Addison's disease; pigmentation and vitiligo (mother and daughter)
{09372} Addison's disease, face
{09373} Addison's disease, buccal pigmentation
{49438} Addison's disease, pigmentation
{49439} Addison's disease, pigmentation
{49440} Addison's disease, atrophy of the adrenal gland
SELECTIVE HYPOALDOSTERONISM is rarely due to primary disease of the adrenal cortex. (Clinicians talk about "hyporeninemic hypoaldosteronism".)
Much more often, the problem is really that the JGA is not producing renin (REE-nin, remember?). Usually the problem is diabetic arteriolar disease (no surprise); less often, it is one of the diseases of the renal tubules and/or interstitium.
* These patients have type IV renal tubular acidosis, exhibit normal response to ACTH stimulation testing, and need a prescription for oral 9α-fludrocortisone.
ACUTE HYPOADRENOCORTICISM ("adrenal apoplexy", "Addisonian crisis"): Sudden collapse, often fatal (the mechanisms are not fully understood, but it involves opening of the peripheral vasculature and shock with high cardiac output; consider giving any such patient glucocorticoid: Arch. Surg. 128: 673, 1993.) The same has been noted to keep blood pressure up in the brain-dead who are being maintained for organ removal (Anesthesiology 112: 1204, 2010). Update on how giving a little extra cortisol to all major trauma patients seems to help: JAMA 305: 1201, 2011.
PLEASE DO NOT MISS THIS ONE.
It may result from undiagnosed adrenal insufficiency (iatrogenic, or patients stressed by infection, surgery, or treatment of concurrent myxedema; see for example J. Traum. 32: 94, 1992), or from known Addison's disease when extra glucocorticoids are not provided during stress.
WATERHOUSE-FRIDERICHSEN SYNDROME ("adrenal apoplexy") features hemorrhage, fibrin thrombi, and sometimes necrosis in the adrenals in a setting of sepsis. It's not clear whether death is due to adrenal shutdown, but it's not helping.
This occurs when there is overwhelming sepsis with hemorrhage into, and destruction of, the adrenals. Patients develop purpura, shock, and die in a few hours.
The etiologic agent is classically the meningococcus, though
staphylococci![]() (possible new
WF-producing strain NEJM 353: 1245, 2005), pneumococci, and H.
influenzae are other important causes (J. Clin. Path. 57: 208, 2004).
(possible new
WF-producing strain NEJM 353: 1245, 2005), pneumococci, and H.
influenzae are other important causes (J. Clin. Path. 57: 208, 2004).
W-F is not rare, and is often overlooked. One group suggests that if your patient in shock does NOT have elevated serum cortisol, he or she presumably has W-F. Draw blood, then give 200 or 300 mg of hydrocortisone (West. J. Med. 150: 582, 1989). Another protocol, for anybody who's septic: Am. J. Med. 98: 266, 1995.
We are now recognizing adrenal insufficiency in very-low-birth-weight preemies as a cause of circulatory collapse. Salivary cortisol screens for this and a bit of glucocorticoid supplementation helps a lot (J. Clin. Endo. Metab. 97: 890, 2012; Ped. Clni. N.A. 58: 1083, 2011).
{24606} Waterhouse-Friderichsen adrenal, gross
{09224} adrenal hemorrhage, consistent with Waterhouse Friderichsen
{07570} adrenal hemorrhage, gross, consistent with Waterhouse-Friderichsen syndrome
|
CUSHING'S SYNDROME: too much glucocorticoid. Review NEJM 332: 791, 1995.

1. IATROGENIC (the most common cause nowadays, preventable in part by giving "alternate-day" glucocorticoid therapy). Of course, the adrenals will be atrophic if glucocorticoids were administered, hyperplastic if ACTH was administered.
2. ACTH-PRODUCING PITUITARY LESION, usually a basophilic microadenoma ("Cushing's disease", "pituitary Cushingism")
The adrenals will usually be diffusely enlarged, but may be nodular, often with one or more large nodules (CT scanners take note -- a single "adenoma" does NOT necessarily rule out the need for pituitary surgery).
"Nelson's syndrome" -- rapid enlargement of the pituitary adenoma leading to hyperpigmentation, blindness and death -- followed adrenalectomy in many of these patients. (Why? It still happens -- sometimes the only way to relieve Cushingism is to remove the adrenals.)
3. ADRENAL CORTICAL ADENOMA OR CARCINOMA ("adrenal Cushingism"); the tumor may be primary, or an autonomous adrenal tumor may develop after years of "pituitary Cushingism")
4. ACTH- (OR CRH-) PRODUCING CANCERS OF OTHER ORGANS: oat-cell carcinoma (very well-known), bronchial and thymic carcinoids (rather common; Ann. Thor. Surg. 94: 1823, 2012; Mayo Clin. Proc. 69: 594, 1994; Ann. Thor. Surg. 95: 1797, 2013), medullary thyroid carcinoma, islet cell cancer; other APUDomas. Full-blown Cushingism is rare in oat cell patients, only because they don't live long enough.... Again, the hyperplasia is usually diffuse but may be nodular.
{49441} looks like an oat cell case; adrenal cortex is hyperplastic, and bears a metastasis
* Urocortin is a hormone widely distributed in the nervous system, with CRH-like activities; it is presently in search of a disease. Both CRF and urocortin are potent anorectic agents. The newly-discovered "urocortin 3" is also called "stresscopin". The truly hardcore can see J. Clin. Endo. Metab. 90: 4671, 2005.
5. Really "primary" adrenal hyperplasia (not due to excess ACTH):
* Genetic syndrome with too many cortisol receptors, low plasma cortisol: J. Clin. Endo. Metab. 85: 14, 2000.
* 6. Cushingism with a burst of cortisol after eating: inappropriate expression of GIP receptors on the adrenal cortex / adrenal adenoma (NEJM 327: 974, 1992; J. Clin. End. Metab. 81: 3168, 1996; J. Clin. Endo. Metab. 86: 583, 2001). There are other aberrant receptor problems as well: J. Clin. Endo. Metab. 88: 416, 2003.
* 7. Recurrent cushingism of pregnancy: Nobody understands it; the adrenal cortex must over-respond to some non-ACTH hormone made during gestation J. Clin. End. Metab. 81: 15, 1996; Clin. Endo. 54: 277, 2001.
Both Cushing's disease and glucocorticoid-secreting adenomas are most common in women ages 15 to 45, but can hit anybody, anytime. (* Cushingism in kids and teens: NEJM 331: 629, 1994).
Symptoms and signs that should alert you to possible Cushingism:
{09367} Cushingism, face
{09370} Cushingism, face
{16109} Cushing's syndrome
{16110} Cushing's syndrome
{16112} Cushing's syndrome "before"
{16111} Cushing's syndrome "after"
{49426} Cushingism, 40 y/o patient
{49427} Cushingism
{49428} Cushingism, hyperplastic adrenal cortex
* Future pathologists: Heavy negative feedback on pituitary basophilic ACTH-producing cells produces "Crooke's hyaline change".
PRIMARY HYPERALDOSTERONISM ("low-renin hyperaldosteronism"): too much mineralocorticoid (review: Postgrad. Med. 95(4): 199, March 1994; NEJM 339: 1820, 1999; Lancet 353: 1341, 1999; Surg. Clin. N.A. 84: 887, 2004; Lancet 371: 1921, 2008)
This results from "idiopathic" adrenal hyperplasia, or an adrenal adenoma.
This is important as a cause of surgically-correctable high blood pressure. Maybe 0.5% of hypertensives have primary hyperaldosteronism. The most recent work (Lancet 2008) uses the retrospectoscope to show that Conn's is probably not so common as has recently been claimed, but still an important concern.
Classically, patients exhibit hypokalemia, alkalosis, and low renin, and a failure of plasma aldosterone levels to increase significantly when the patient goes from supine to standing position.
Surprisingly, these patients do not have edema. (The effects of aldosterone in hanging onto body salt is overridden by atrial natriuretic peptide.)
Low potassium is likely to cause muscle weakness, and even paralysis.
Trap: These patients can die from hypokalemia if you give them thiazide diuretics to treat their high blood pressure.
Today, we screen by looking for the plasma aldosterone / plasma renin activity ratio. Some hypertensives have elevated levels, and many of these people will indeed have an aldosteronoma that can be removed (Am. J. Med. Sci. 324: 227, 2002); the rest will usually have hyperplastic cortices.
The most familiar cause is an "autonomous" adrenal cortical adenoma (CONN'S SYNDROME), often very small. It produces aldosterone (rare Conn-omas produce DOC instead). You'll clinch the diagnosis by sampling aldosterone levels in the adrenal veins (J. Clin. End. Metab. 86: 1066, 2001). Surgery is curative (Ann. Surg. 219: 347, 1994; Postgrad. Med. 95(4): 199, Mar. 1994); it is now routinely done via laparoscope (review J. Urol. 169: 32, 2003), and those that look harmless on scan may even be removed by partial adrenalectomy (J. Urol. 184: 28, 2010).
For "bilateral adrenal hyperplasia", medical treatment is the norm, or if sampling the adrenal veins reveals one gland to be producing much more aldosterone than the other, removing that gland usually works (J. Am. Coll. Surg. 213: 106, 2011).
![]() Adrenal cortical adenoma
Adrenal cortical adenoma
Produced Conn's
Wikimedia Commons
The rest of the patients have "idiopathic hyperaldosteronism", with normal or hyperplastic adrenals. These patients get spironolactone. Not surprisingly, the borderland between these and the adenomas is blurry (Surgery 106: 1161, 1990); probably it's best to operate only if the hypertension is unsuppressible medically like a Conn-oma.
A few patients have glucocorticoid-correctable hyperaldosteronism and hypertension. This is transmitted autosomal-dominant. It is now clear that the problem is a chimeric beta-hydroxylase/aldosterone synthetase gene (Nature 355: 262, 1992; Lancet 339: 1024, 1992; screening kids Arch. Dis. Child. 71: 40, 1994; J. Urol. 154: 510, 1995). Update J. Clin. Endo. Metab. 87: 3187, 2002. When the cell is told by ACTH to make cortisol, it pumps out huge amounts of aldosterone, too. (Thinkers: Giving a tiny amount of exogenous glucocorticoid solves the problem. How? If you can't answer this, go back and review your endocrine physiology.)
* Hypertension from a mutated aldosterone receptor stuck in the "on" position: Science 289: 119, 2000.
* Another cause is "apparent mineralocorticoid excess syndrome", a lack of 11-β-hydroxysteroid dehydrogenase type 2, which turns cortisol to cortisone in the renal tubules; cortisol ends up overstimulating the mineralocorticoid receptors. The forme fruste may be a common contributor to "idiopathic" low-renin hypertension even with normal potassium. See J. Clin. Endo. Metab. 86: 1247, 2001; Lancet 353: 1341, 1999. Yet another is a 21-deoxyaldosteronoma (J. Clin. End. Metab. 80: 737, 1995). Rarely an ovarian cancer produces aldosterone (series Arch. Int. Med. 156: 1190, 1996).
SECONDARY ALDOSTERONISM is much more common. It is part of the picture in CHF, cirrhosis, nephrotic syndrome, Goldblatt hypertension, and other common problems.
Don't forget Bartter's hypokalemia (vessels are insensitive to angiotensin and/or the sodium pump in the ascending loop of Henle doesn't work -- Hosp. Pract. 29(5): 103, 1994.)
Don't confuse this with salt-retaining congenital adrenal hyperplasia (see below).
CONGENITAL ADRENAL HYPERPLASIA: autosomal-recessive virilization syndromes that, in their most severe forms, affect young children.
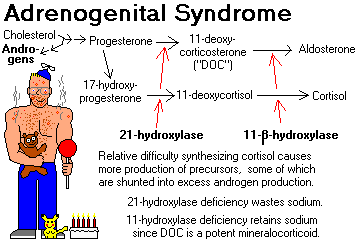
Deficiencies (mild or severe) of the various enzymes required to synthesize cortisol result in decreased production of cortisol and other hormones.
This results in increased ACTH, with resulting adrenal cortical hyperplasia.
Steroid precursors are shunted into the production of abnormally large amounts of the androgen androstenedione (ambiguous genitalia in girls, "infant Hercules" and Leydig cell nodules in boys, etc.)
Remember these two types (there are at least six others):
21-HYDROXYLASE DEFICIENCY (most common): no cortisol, aldosterone, or DOC, hence salt wasting. This gene is inside the HLA locus. Review J. Clin. Endo. Metab. 88: 2624, 2003.
11-BETAHYDROXYLASE DEFICIENCY: huge amounts of DOC, causing salt retention and high blood pressure (molecular biology of the gene Proc. Nat. Acad. Sci. 90: 4552, 1993).
Full-blown congenital adrenal hyperplasia is a devastating illness, especially for women. Mild variants of these syndromes (i.e., relatively ineffective enzymes -- especially 21-hydroxylase deficiency) are probably widespread -- causing, for example, amenorrhea in girls or hirsutism in older women.
It's important to find these people because a little dexamethasone given daily will greatly improve the internal milieu.
To test your female patient with amenorrhea or hirsutism, administer ACTH and measure plasma 17-hydroxyprogesterone one hour later. It will be elevated if your patient has even mild 21-hydroxylase deficiency.
{49437} adrenogenital syndrome 2 year old girl
{24450} adrenogenital syndrome, virilized baby girl
{49432} 11-hydroxylase deficiency, 11 month old boy
ADRENAL CORTICAL ADENOMAS
These are round, yellow (like the adrenal cortex) nodules. (* Purists call them "nodular hyperplasia" if the surrounding cortex is at all lumpy-bumpy).
Adrenal cortical adenomas are surprises at around 2% of autopsies and abdominal CT scans. They can cause Cushing's syndrome, Conn's syndrome, or virilization -- but the vast majority seem to produce nothing.
They are commonly discovered on CT scans too, and clinicians are learning to ignore small adrenal masses ("incidentalomas", so long as there is no evidence of steroid or catecholamine over-production).
Consider screening folks with incidentalomas for Cushingism. Maybe 20% of these really are active, and contribute to hypertension, diabetes, obesity, and osteoorosis, even if the patient is not floridly Cushingoid. Common sense triumphant. See Surg. Clin. N.A. 84: 875, 2004. "Occult/subclinical Cushingism due to incidentaloma" is now a recognized entity: J. Clin. Endo. Metab. 88: 5808, 2003. NIH Consensus Conference: Ann. Int. Med. 138: 424, 2003.
A question that is NOT settled and is interesting is whether removing a little adrenal cortical incidentaloma will relieve "features of mild hypercortisolisim" (i.e., when the dexamethasone suppression test / late-night salivary cortisol levels / 24 hour urinary cortisol don't show real Cushingism -- but your patient is still fat / glum / hypertensive / glucose intolerant). See J. Clin. Endo. Metab. 95: 4106, 2010.
As above, adrenal masses are generally not biopsied prior to excision. The case against fine-needling incidentalomas, made by surgeons: Surgery 142: 497, 2007.
* Leave discussions of the arcana of these common lesions to us. This includes "spironolactone bodies" (pink scroll-like things, also found in the ZG of folks taking spironolactone), "black adenomas", and much more.
"Adrenal cysts" seen on scans have unpredictable pathology: Cancer 101: 1537, 2004.
{09220} adrenal cortical adenoma, gross
{20312} adrenal cortical adenoma, gross
{49436} adrenal cortical adenoma, gross; this one produced Conn's syndrome
{10298} adrenal cortical adenoma
{20315} adrenal cortical adenoma, histology
{09221} adrenal cortical adenoma, histology
{09222} adrenal cortical adenoma, histology
{08964} adrenal cortical adenoma, histology (hard to tell from normal cortex)
{09052} adrenal cortical adenoma, electron micrograph; note tubular cristae in mitochondria
(spaghetti instead of lasagna)
{09375} effect of masculinizing adrenal cortical adenoma, "before"
{09374} effect of masculinizing adrenal cortical adenoma, "after"
{49434} gynecomastia in five-year old boy, feminizing adrenal cortical adenoma
|
|
ADRENAL MYELOLIPOMA: a metaplasia-choristoma made of bone marrow. They can be big but are generally harmless. You can see bone marrow in adrenal cortical hyperplasia too.
{25412} adrenal myelolipoma, gross
{49443} adrenal myelolipoma, gross
{25413} adrenal myelolipoma, histology
![]() Adrenal myelolipoma
Adrenal myelolipoma
Pittsburgh Pathology Cases
ADRENAL CORTICAL CARCINOMA (Am. J. Clin. Path. 105: 76, 1996; J. Urol. 169: 5, 2003; J. Clin. Endo. Metab. 95: 4812, 2010)
These are rare cancers that are often lethal. Many are are hormonally active and produce Cushing's, Conn's, and/or virilization.
Future pathologists: These are usually obviously malignant, grossly and microscopically, with ten or more mitotic figures per 10 high power fields.
The most reliable way of spotting malignancy is still the mitotic figure count; other systems have not proved very reliable (Am. J.Clin. Path. 127: 398, 2007).
* Mixed endocrine syndromes usually mean cancer. Adrenal tumors that feminize, or that produce androgens without glucocorticoids, are most often malignant.
Today's surgeons are operating these cancers in the hope of cures, and there are enough cases now with good results that it's possible to demonstrate the value of taking lymph nodes (Ann. Surg. 255: 363, 2012) and of removing single lung metastases (Ann. Thorac. Surg. 92: 1965, 2011).
Mitotane, an analogue of the old-fashioned insecticide DDT, is the classic mainstay of therapy. (It will also destroy and scar any remaining normal adrenal gland.)
* Future pathologists: We used to use the electron microscope to find tubulovesicular mitochondria, which are characteristic of adrenal cortex to confirm that something was really an adrenocortical carcinoma. SF1 stain ("steroidogenic factor 1") is sensitive and specific (J. Clin. Endo. Metab. 95: E161, 2010) for adrenal cortical carcinoma (really, for origin in a steroid-producing cell) if you are in doubt. Criteria for malignancy have been developed; since this cancer is not particularly treatable, their usefulness is limited.
{24087} adrenal cortical carcinoma, gross
{40196} adrenal cortical carcinoma
{24090} adrenal cortical carcinoma, histology
NOTE: Cancer in the adrenals is usually metastatic carcinoma. Half of all lung cancers eventually metastasize to the adrenals. Adrenal insufficiency sometimes results when replacement is massive, but is usually missed clinically.
![]() Metastatic cancer in the adrenals
Metastatic cancer in the adrenals
WebPath Photo
![]() Cancer metastatic to the adrenal
Cancer metastatic to the adrenal
AFIP
Wikimedia Commons
THE ADRENAL MEDULLA: "An organ not essential to life".
Around 10% of the normal adrenal by weight. The source of "adrenalin" (epinephrine, also norepinephrine). At autopsy it is gray, unless it has autolyzed. (The old name "adrenal capsules", from the pre-refrigeration era, reflects the fact that the medulla had usually liquified by the time the anatomists and pathologists got to it.)
"Adrenal medullary hyperplasia" (i.e., the medulla is too big; one way to tell is that it extends into the tail, where it's not supposed to go) is a marker for MEN II and a few rarities. It can be diffuse or nodular, and it may be best to call any nodule bigger than 1 cm a pheochromocytoma.
The only bona-fide diseases are two tumors that may arise here or at the other chromaffin tissue masses -- pheochromocytoma (well-differentiated, adults) and neuroblastoma (poorly-differentiated, children).
* The story of the old adrenal-to-brain transplant for parkinsonism: Mayo Clin. Proc. 65: 305, 1990.
PHEOCHROMOCYTOMA ("paraganglioma", "pheo", formerly "ten percent tumor"; big NIH consensus review Ann. Int. Med. 134: 315, 2001; big review for pathologists Arch. Path. Lab. Med. 132: 1272, 2008).
This tumor is named for its colorful reaction in fixatives containing chromic acid salts.
Pheochromocytomas secrete norepinephrine (most common) and/or epinephrine (usually less, * and often other things, including dopamine, serotonin, ACTH, somatostatin, neuropeptide Y, and/or VIP; Cancer Res. 49: 7010, 1990).
Pathologists confirm that a likely-looking tumor is a pheochromocytoma by staining it up for chromogranin and/or synaptophysin.
The infamous paroxysms of extreme hypertension, accompanied by sweating, headache, and other autonomic disturbances, probably result from physical compression and/or ischemia of the "pheo".
Even a tiny (1 gm) benign pheochromocytoma can make a person very sick and will eventually cause death.
Today, "pheochromocytoma" is defined to arise in the adrenal medulla. Similar tumors (less common) arising elsewhere are called "extra-adrenal paragangliomas." These sites include the organs of Zuckerkandl ("para-aortic bodies", i.e., the little nubbins of chromaffin tissue at the origin of the inferior mesenteric artery and/or aortic bifurcation -- prominent and surely important in the unborn child, but regressed by birth), paravertebral sympathetic chain, urinary bladder (patients get terrible headaches whenever they urinate), or "paraganglia" such as the carotid body.
The old business about "ten percent of pheo cases involve both adrenals, 10% are familial, and 10% metastasize" is history. Today, everything's about the new genetics.
* "Composite pheo" contains some neuroblastoma, ganglioneuroblastoma, or nerve-sheath tumor; think of MEN II or neurofiromatosis. If there's no neroblastoma, the presence of other elements is probably not a bad prognostic indicator. Update Am. J. Clin. Path. 132: 69, 2009.
You will often be reminded of the MEA ("MEN", "multiple endocrine adenoma/neoplasia syndromes") -- common autosomal dominant conditions that predispose patients to certain endocrine tumors. Pre-natal diagnosis is available for these tumor-gene syndromes. Learn them now:
MEN I: PPP (Wermer's syndrome), gene MENI, protein menin * on 11q13; * less often the gene CDKN1B
Parathyroid adenoma(s) (1 or, often, more glands) / "chief cell hyperplasia" (i.e., all four glands): NEJM 321: 213, 1989).
Pituitary adenoma (anterior)
Pancreatic islet cell adenoma (gastrinoma)
* The pituitary-and-parathyroid-only variant usually is from a different locus that remains to be discovered: J. Clin. Endo. Metab. 92: 1948, 2007.
MEN IIa: PAC (Sipple's syndrome); the RET gene (Nature 367: 315, 375, 377 & 378, 1994; NEJM 335: 943, 1996; J. Clin. End. Metab. 81: 2711, 1996; screening for the gene J. Clin. Endo. Met. 78: 1261, 1994 and Mayo Clin. Proc. 72: 430, 1997; new alleles keep appearing J. Clin. Endo. Metab. 89: 4142, 2004; surveillance J. Clin. End. Metab. 82: 897, 1997).
Parathyroid adenoma(s) (1 or, often, more glands) / "chief cell hyperplasia"
Adrenal medullary tumor (pheochromocytoma) or hyperplasia
Calcitonin-producing hyperplasia-carcinoma of thyroid
MEN IIb (MEN III; * eponyms like Gorlin-Vicker haven't caught on):
Similar to MEN IIa; the patients have Marfanoid body habitus and mucosal (ganglio)neuromas (bumps on the edges of their tongues and elsewhere), and are less likely to have parathyroid problems. Same locus, different allele (Nature 1994, see above.)
Grossly, pheos are very bloody (because they are very vascular), and often show fibrosis, calcification, cystic change, or even * fatty change (?!)
Microscopically, pheos resemble adrenal medulla.
Special stains are available that show norepinephrine and/or epinephrine in granules (* future pathologists: aqueous fixation washes them out.)
* Nowadays, a tumor that stains for phenylethanolamine-N-methyltransferase (PNMT) has the "adrenergic phenotype", one that doesn't has the "noradrenergic phenotype".
Of course, that's history now. Pheos light up with chromogranin A and tyrosine hydroxylase. Extra-adrenal paragangliomas light up with chromogranin A but often not with tyrosine hydroxylase.
For pheos, the traditional teaching has long been that there are no histologic criteria for malignancy, not even vascular invasion. The honest pathologist cannot predict the tumor's behavior.
To prove malignancy you must find pheo in a location where it could not have arisen. Five-year survival rate with malignant pheo is around 50%.
* "Pheo balls" are hyaline spheres that can be very big. You can see them in most normal medullas if you look hard enough. They must be thanatosomes. Fun to know: They are acid-fast and autofluorescent!
* A pheo or neuroblastoma, as a frozen section, exposed to formalin, fluoresces yellow-green from the catecholamines getting altered. This is a helpful study that pathologists can perform immediately on retrieval of the tumor.
Regardless of location and appearance, the patients will report anxiety, headache, palpitations, "panic attacks", sweating, dizziness, etc. (Again, you may suspect the basic problem is emotional. "Pheo is a great imitator.")
"Textbook" pheochromocytoma patients have paroxysms of severe hypertension. Actually, the majority show sustained high blood pressure.
Pheochromocytoma is present in fewer than 1% of people with high blood pressure, but it's a diagnosis you don't want to miss.
Pheos are still often missed clinically (Am. J. Surg. 179: 212, 2000) and are all-too-familiar surprises at autopsy (Lancet 335: 1189, 1990). The patients typically had been told they had "benign essential hypertension" and "emotional problems".
In addition to causing bad high blood pressure and all that goes with it, high levels of circulating catecholamines can directly (and likely permanently, as with cocaine) damage the myocardium can cause coronary spasm, and can play havoc with smooth muscle (renal arteries, bowel, brain, etc. -- angiographers may report "vasculitis".)
Screening tests for pheos detect increased amounts of catecholamines or their metabolites in blood or urine.
The classic screen for pheos (24 hour urinary vanillylmandelic acid -- VMA) is being superseded by more sensitive and specific tests.
Today's "most sensitive screen" is the plasma free metanephrine assay (Ann. Int. Med. 134: 315, 2001; Arch. Int. Med. 160: 2521, 2000; JAMA 287: 1427, 2002).
There's a radioisotope scanner/treatment for pheo and neuroblastoma -- I-131 labeled metaiodobenzylguanidine (MIBG); it's not very sensitive for diagnosis (J. Clin. Endo. Metab. 86: 685, 2001). Today, the 6-[18F]fluorodopamine PET scan seems to be preferred for spotting metastatic pheochromocytoma.
* Finding the hidden pheo using novel techniques, including 6-[18F]-fluorodopamine: J. Clin. Endo. Metab. 86: 3641, 2001.
* The Mayo crew examines how much it would cost to screen every hypertensive patient for pheo using each of three methods, and simply states that spending $50,000 or $100,000 per patient found isn't worth it (J. Clin. Endo. Metab. 89: 2859, 2004); I am not sure I agree, and certainly you need to work up the young ones, the ones with headaches, and the ones with "nerves".
Treatment is surgical, with very careful management of fluid status and blood pressure before and after surgery (J. Urol. 161: 764, 1999). The anesthesiologist, of course, has an extra challenge (Anesth. Analg. 91: 1118, 2000). And surgeons must be careful manipulating the tumor! Adrenal sparing surgery, i.e., let's leave a bit of cortex behind: Br. J. Surg. 86: 94, 1999. Today, it's likely that the tumor will be removed successfully via the laparoscope (Urol. Clin. N.A. 28: 97, 2001; update Arch. Surg. 145: 893, 2010).
* Injecting epinephrine to fake a pheo: JAMA 266: 1553, 1991 (weird!).
{20316} pheochromocytoma in adrenal, gross
{25417} pheochromocytoma, gross
{49444} pheochromocytoma, gross
{09226} pheochromocytoma, gross
{09227} pheochromocytoma, showing positive brown staining with chromic acid ("chromaffin")
{08874} pheochromocytoma, histology
{08873} pheochromocytoma, histology
{25418} pheochromocytoma, histology
{09228} pheochromocytoma, histology
{09229} pheochromocytoma, histology, positive chromaffin reaction
{09080} pheochromocytoma, electron micrograph showing granules
{09081} pheochromocytoma, electron micrograph showing granules
{08056} pheochromocytoma cardiotoxicity
{08053} pheochromocytoma cardiotoxicity; note contraction bands
NEUROBLASTOMA (Ped. Clin. N.A) 55: 97, 2008; genes JAMA 307: 1062, 2012)
Either Wilms tumor or neuroblastoma is the most comon extracranial solid cancer of children. It is derived from primitive nerve elements (* and the cells will always grow neurites, at least in tissue culture). Discovered by Virchow.
A majority of neuroblastomas arise in or near the adrenals.
They seem to strike at random. * Three risk loci, all at 6p22, have been spotted (NEJM 358: 2585, 2008); an autosomal dominant allele of ALK produces neuroblastoma when it amplifies itself (Nature 455: 930, 2008).
Grossly, neuroblastomas are soft, white tumors.
Portions often undergo dystrophic calcification (which helps the radiologist make the diagnosis.)
The tumor eventually metastasizes widely. "Blueberry muffin baby" is a repulsive, classic description for a neuroblastoma patient with multiple skin metastases.
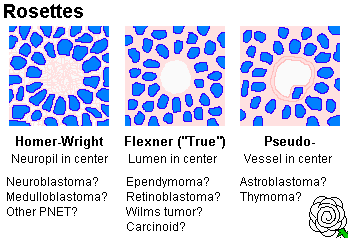
Histologically, neuroblastoma is a tumor of "small blue cells" (i.e., cancers with small, fairly-uniform cells with scanty cytoplasm). Often (but not always) the cells are arranged in "rosettes" (recalling neural tubes) around a tangle of neurites (* "Homer Wright rosettes", pretty-much diagnostic). EM shows neurosecretory granules and often neurites too. (* True "Flexner" rosettes surround a hole. Think of ependymomas or retinoblastomas.)
* Future pathologists: Immunohistochemistry helps differentiate this from other "tumors of small blue cells" (the LEMON family), and will also help you recognize their nondescript cells in bone marrow. The better-differentiated neuroblastomas are likely to stain positive for neuron specific enolase, S-100, and/or synaptophysin, but the more primitive ones will be negative for everything. In tissue culture, neuroblastoma cells sprout neurites almost at once.
Grading of the tumor is based on mitotic figure counts, with karyorrhexis being taken into account nowadays also: Cancer 77: 1582, 1996 updates the older Shimada system which was based on how much stroma was present and whether the cells were maturing (i.e., differentiating as neurons with more cytoplasm.) The update is the "International Neuroblastoma Pathology Committee" system (Cancer 86: 364, 1999; Cancer 92: 2451 & 2699, 2002; Cancer 94: 1574, 2002). "MKI" is the "mitosis-karyorrhexis index", i.e., what percent of cells exhibit these; more than 4% is bad, less than 2% is good. The systems for distinguishing "favorable" and "unfavorable" histopathology now incorporate stroma, pattern, and maturation as well as the child's age but MKI is still central (J. Clin. Onc. 27: 289, 2009).
Most neuroblastomas produce catecholamines, resulting in elevated urinary metabolites. (* They also produce certain characteristic protein markers, etc., etc.)
The classic test involves checking urine for homovanillic acid (HVA) and vanillylmandelic acid (VMA). Japan screens all babies at six months of age (Lancet 2: 152, 1988); skeptical Brits suggest that they find only regressing tumors (Lancet 337: 344, 1991). The Germans have screened their babies at one year and find it lot of cases, but I couldn't tell whether they're saving lives (J. Clin. Onc. 17: 1200, 1999 -- anybody want to do a lab presentation on this?). Now the Japanese are doubting whether they've saved any lives, either (Canc. Caus. Contr. 9: 631, 1998; "we haven't" J. Ped. Surg. 37: 949, 2002). Neuroblastoma screening has not caught on in the US in the "managed care era"; both big studies indicated it's a bad idea NEJM 346: 1041 & 1047, 2002.
There are also a variety of curious (probably autoimmune) paraneoplastic syndromes that result from neuroblastomas, including neurodegenerative disorders similar to those in oat-cell carcinoma.
Prognosis in neuroblastoma:
And of course, it's good if the cells are differentiating...
In a baby, the tumor is likely to regress/differentiate/mature to a "differentiating neuroblastoma" (more than 5% of the cells look like big ganglion cells, and/or some schwann-cell-like stroma), a stroma-rich GANGLIONEUROBLASTOMA (a neuroblastoma, probably with lots of big ganglion cells, with 50% or more being a stroma that looks like schwann cells) or a thoroughly benign GANGLIONEUROMA; Br. J. Surg. 83: 263, 1996). Ganglioneuromas are made of ganglion-like cells in a fibrous "schwannian" stroma; it may occasionally appear in adults (J. Clin. Endo. Metab. 95: 3118, 2010; Surgery 147: 854, 2010; Surgery 149: 99, 2011). In maybe 2% of autopsies on infants dying of unrelated causes, there is a neuroblastoma-like "incidentaloma". Obviously most of these cure themselves. We wish we knew exactly why/how this happens.
* The process begins with the appearance of S100-positive Schwann cells, which are from outside the tumor. If the tumor is to self-cure, it must be near-triploid and have intact chromosome 1 (NEJM 334: 1505 & 1537, 1996).
{24716} neuroblastoma, histology, good rosettes
{25420} neuroblastoma, gross
{25422} neuroblastoma, histology
{39049} neuroblastoma, gross; probably an incidental finding in a newborn
{09009} neuroblastoma, histology
{09232} neuroblastoma, histology
{20046} neuroblastoma, histology
{20047} neuroblastoma, histology
{09011} neuroblastoma, histology, good rosettes
{08963} neuroblastoma histology (sorry, no good rosettes)
{25424} ganglioneuroblastoma, histology
{25426} ganglioneuroblastoma, histology
{24608} ganglioneuroma, gross
![]() Neuroblastoma patient & family
Neuroblastoma patient & family
Cindie and 10 y/o Derek Madsen
Pulitzer-Prize photoessay
In toddlers, spontaneous remission is less likely, but even metastatic disease is often cured by chemotherapy.
In older kids and adults, neuroblastomas grow slower but seldom self-cure or respond well to therapy (Cancer 79: 2028, 1997). We are getting good results even in the more difficult cases nowadays thanks to I131-MIBG (Cancer 117: 4286, 2011).
Retinoblastomas (cones of the eye), medulloblastomas (cerebellum), pinealoblastomas (pineal), and adult neuroblastomas (lots of places -- many of these are probably Ewing variants) are related pediatric tumors that look like neuroblastomas microscopically.
* The tendency today is to call these "primitive neuroectodermal tumors", despite obvious differences in their basic biology.
* Neuroblastoma of the olfactory epithelium is a special entity. Don't worry about it.
INTRODUCTION TO ADRENAL TESTING
The aphorism -- "A physician is only as good as his/her index of suspicion" -- is especially applicable to endocrine disease. As an alert clinician, you will often suspect adrenal gland problems and will want to order sensitive tests.
Some classic cases:
If the morning serum cortisol is >13 mcg/dL, you are probably not dealing with addisonism. If the morning serum cortisol is <=13 mcg/dL, order a rapid ACTH ("cosyntropin", "synacthen") stimulation test.
After 30-60 minutes, the serum cortisol should spike to at least 500 nanomoles/Liter = 18 micrograms/dL.
If it doesn't, you have adrenal insufficiency, either primary (the glands are diseased) or secondary (the glands atrophied from lack of ACTH stimulation).
In the past, we used to give an ACTH stimulation test each day for a week; patients with secondary adrenal insufficiency would grow their adrenals back from the test (and probably want to come back for more ACTH!)
Nowadays, ACTH plasma assays are good enough to make the distinction easier. Injected ACTH clears from the blood in a few minutes. Nowadays, use the 30-minute blood sample to assay not just cortisol, but also serum ACTH (it'll probably still be high in primary adrenal disease, low-ish in pituitary insufficiency). Also check serum aldosterone in the same sample; the ability of the adrenals to produce aldostone isn't lost as much as the ability to produce cortisol when the problem is in the pituitary gland. A lack of a normal spike after ACTH administration confirms primary adrenal insufficiency.
A "spot serum cortisol" is worthless (the stress of venipuncture can cause a false negative.) A "24 hour urine cortisol" is worthless (it can be zero in health.) A "spot serum ACTH" is worthless. It's secreted in pulses.
Order serum cortisol determinations at 8 AM and 8 PM (circadian rhythm is always lost in Cushingism), plus a low-dose dexamethasone suppression test, OR just order one (or maybe two or three) 24 hr urinary free cortisol assays. The most convenient screen now being promoted is a late-night salivary cortisol (J. Clin. Endo. Metab. 94: 456, 2008).
Screen with a serum aldosterone/renin ratio. If high, measure urine aldosterone on a high-salt diet AND/OR see whether you can suppress the aldosterone using fludrocortisone AND/OR check to see if plasma aldosterone fails to increase on standing up AND/OR consider performing a saline infusion aldosterone-suppression test AND/OR consider a CT scan. Nobody really knows what's best. Current thinking: J. Clin. Endo. Metab. 91: 2618, 2006.
Administer ACTH and measure blood 17-OH-progesterone.
Check serum/urine catecholamines and/or metabolites, ask your lab.
Of course, only some of these patients are endocrine cases. As a rule, meaningful hormone assays are performed under conditions of attempted stimulation (if you suspect deficiency) or attempted suppression (if you suspect over-production). If your screening tests are positive, or if you have any doubts, get consultation.
If you diagnose endocrine disease that is not present, the patient gets lifelong medication, unnecessary surgery, or unnecessary radiation. If you fail to diagnose a disease that is present, the patient is likely to die of a disease that might have had an excellent prognosis. (Suicide is common among patients with untreated Cushing's syndrome.) If you make the correct diagnosis, the treatment of endocrine disease is very satisfying to physician and patient alike -- because it works.